Analysis of a Mixture of Carbonate and Bicarbonate
advertisement

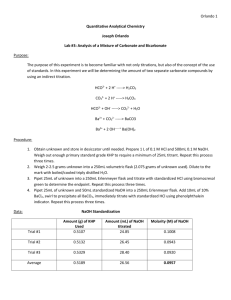
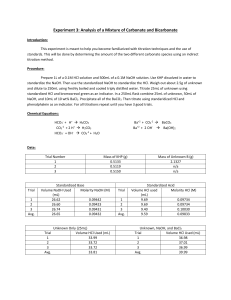
Analysis of a Mixture of Carbonate and Bicarbonate Purpose: With the use of secondary standards, which are obtained from the primary standard. With these we will be determining the amount of two different carbonate species using indirect methods of titration. Procedure: Obtain and unknown carbonate samples which contains the two different carbonate species, store this in a decanter until ready to use. Standardize a base of about 0.1M NaOH using KHP. Then using this standardized base standardize a acid of about 0.1 M HCl. Weigh about 2.0-2.5g of unknown into a 250mL volumetric flask and dilute with freshly boil and cooled tap water. Pipet a 25.00mL aliquot of your unknown into a 250mL Erlenmeyer and titrate using the standardized HCl. Use bromocresol green to determine endpoint. Then pipet a 25.00mL aliquot of unknown and a 50.00mL aliquot of standard NaOH into a 250mL Erlenmeyer. Add 10mL by wt% BaCl2 to precipitate out BaCO2, then titrate using the standard HCl and phenolphthalein indicator. Report relative percent carbonate and bicarbonate in unknown and standard deviation values. Chemical Equations: HCO3- + H+ H2CO3 CO3-2 + 2 H+ H2CO3 HCO3- + OH- CO3-2 + H2O Ba+2 + CO3-2 BaCO3 Ba+2 + 2 OH- Ba(OH)2 Data: Trial Number 1 2 3 Mass of Unknown B (g) = 2.3672g Trial 1 2 3 Avg. Mass of KHP (g) 0.5216 0.5137 0.5258 Standardized Base Volume NaOH Used Molarity NaOH (M) (mL) 26.61 0.0959 27.50 0.0915 25.60 0.1006 0.096 Trial 1 2 3 Avg. Unknown Only (25mL) Volume HCl Used (mL) 32.63 31.63 31.98 32.08 Trial 1 2 3 Avg. Standardized Acid (10mL) Volume NaOH Molarity HCl (M) used (mL) 10.30 0.09888 10.30 0.09888 10.30 0.09888 10.30 0.09888 Unknown, NaOH, and BaCl2 Trial Volume HCl Used (mL) 1 32.50 2 32.75 3 32.85 Avg. 32.70 Calculated Data: Trial Mol CO3&HCO3 1 0.003226 2 0.003127 3 0.003162 Avg Std Dev Mol NaOH Total 0.0048 0.0048 0.0048 Mol NaOH Reacted w/HCl 0.00322 0.00313 0.00316 Mol NaOH Mol CO3 Mass% HCO3 Mass% CO3 0.00165 0.00145 0.00152 40.72 43.72 42.53 42.32 1.511 41.83 36.75 38.53 39.04 2.578 Reacted W/bicarb. 0.00158 0.00168 0.00165 Calculations: Molarity of NaOH: 𝑀𝑁𝑎𝑂𝐻 1 𝑚𝑜𝑙 𝐾𝐻𝑃 1 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 = (𝑚𝐾𝐻𝑃 × × )÷𝑉 204.22𝑔 𝐾𝐻𝑃 1 𝑚𝑜𝑙 𝐾𝐻𝑃 Molarity of HCl: 𝑀𝑁𝑎𝑂𝐻 𝑉𝑁𝑎𝑂𝐻 = 𝑀𝐻𝐶𝑙 ∆𝑉𝐻𝐶𝑙 Moles of Carbonate and Bicarbonate: 𝑀𝐻𝐶𝑙 × 𝑉𝐻𝐶𝑙 𝑎𝑑𝑑𝑒𝑑 = 𝑚𝑜𝑙𝑒𝑠 𝐻𝐶𝑙 𝑚𝑜𝑙𝑒𝑠 𝑎𝑐𝑖𝑑 = 𝑚𝑜𝑙𝑒𝑠 𝐶𝑂3&𝐻𝐶𝑂3 Total moles of NaOH: 𝑀-𝑁𝑎𝑂𝐻 × 𝑉𝑁𝑎𝑂𝐻 = 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 Moles of NaOH reacted with HCl: 𝑀𝐻𝐶𝑙 × 𝑉𝐻𝐶𝑙 𝑎𝑑𝑑𝑒𝑑 = 𝑚𝑜𝑙𝑒𝑠 𝐻𝐶𝑙 𝑚𝑜𝑙𝑒𝑠 𝑎𝑐𝑖𝑑 = 𝑚𝑜𝑙𝑒𝑠 𝑏𝑎𝑠𝑒 Moles NaOH reacted with Bicarbonate: 𝑚𝑜𝑙𝑒𝑠𝑁𝑎𝑂𝐻 𝑤𝑖𝑡ℎ 𝐻𝐶𝑂3 = 𝑚𝑜𝑙𝑒𝑠𝑁𝑎𝑂𝐻 𝑡𝑜𝑡𝑎𝑙 − 𝑚𝑜𝑙𝑒𝑠𝑁𝑎𝑂𝐻 𝑤𝑖𝑡ℎ 𝐻𝐶𝑙 Moles of Carbonate: 𝑚𝑜𝑙𝑒𝑠𝐶𝑂3 = 𝑚𝑜𝑙𝑒𝑠𝐶𝑂3&𝐻𝐶𝑂3 − 𝑚𝑜𝑙𝑒𝑠𝐻𝐶𝑂3 Mass percent of Bicarbonate: 61.016𝑔 𝐻𝐶𝑂3 𝑚𝑜𝑙𝑒𝑠𝐻𝐶𝑂3 × = 𝑚 𝐻𝐶𝑂3 1 𝑚𝑜𝑙 𝐻𝐶𝑂3 𝑚 𝐻𝐶𝑂3 × 100% = 𝑚𝑎𝑠𝑠% 𝑚 𝑠𝑎𝑚𝑝𝑙𝑒/10 Mass percent of Carbonate: 60.008𝑔 𝐶𝑂3 𝑚𝑜𝑙𝑒𝑠𝐶𝑂3 × = 𝑚 𝐶𝑂3 1 𝑚𝑜𝑙 𝐶𝑂3 𝑚 𝐶𝑂3 × 100% = 𝑚𝑎𝑠𝑠% 𝑚 𝑠𝑎𝑚𝑝𝑙𝑒/10 Molarity of NaOH Example: 0.0959𝑀 = (0.5216𝑔 × 1 𝑚𝑜𝑙 𝐾𝐻𝑃 1 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 × ) ÷ .02661𝐿 204.22𝑔 𝐾𝐻𝑃 1 𝑚𝑜𝑙 𝐾𝐻𝑃 Molarity of HCl Example: 0.096𝑀(10.30𝑚𝐿) = 𝑀𝐻𝐶𝑙 (10𝑚𝐿) 𝑀𝐻𝐶𝑙 = 0.0988 Moles of Carbonate and Bicarbonate Example: 0.0988𝑀 × 0.03263𝐿 = 0.00322𝑚𝑜𝑙𝑒𝑠 𝐻𝐶𝑙 = 0.00322𝑚𝑜𝑙𝑒𝑠 𝐶𝑂3&𝐻𝐶𝑂3 Total moles of NaOH Example: 0.09888𝑀 × 0.0500𝐿 = 0.0048𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 Moles of NaOH reacted with HCl Example: 0.09888𝑀 × 0.03263𝐿 = 0.0000322𝑚𝑜𝑙𝑒𝑠 𝐻𝐶𝑙 = 0.00322𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 𝑤𝑖𝑡ℎ 𝐻𝐶𝑙 Moles NaOH reacted with Bicarbonate Example: 𝑚𝑜𝑙𝑤/𝐻𝐶𝑂3 = 0.0048𝑚𝑜𝑙𝑡𝑜𝑡𝑎𝑙 − 0.00322𝑚𝑜𝑙𝑤/𝐻𝐶𝑙 = 0.00158𝑚𝑜𝑙𝑒𝑠𝑁𝑎𝑂𝐻 𝑤𝑖𝑡ℎ 𝐻𝐶𝑂3 Moles of Carbonate for Example: 𝑚𝑜𝑙𝐶𝑂3 = 0.003226𝑚𝑜𝑙𝐶𝑂3&𝐻𝐶𝑂3 − 0.00158𝑚𝑜𝑙𝐻𝐶𝑂3 = 0.00165𝑚𝑜𝑙𝑒𝑠 𝐶𝑂3 Mass percent of Bicarbonate Example: 0.00158𝑚𝑜𝑙𝐻𝐶𝑂3 × 61.016𝑔 𝐻𝐶𝑂3 = 0.0964𝑔 𝐻𝐶𝑂3 1 𝑚𝑜𝑙 𝐻𝐶𝑂3 0.0964𝑔 𝐻𝐶𝑂3 × 100% = 40.72% 2.3672𝑔/10 Mass percent of Carbonate for Example: 60.008𝑔 𝐶𝑂3 0.00165𝑚𝑜𝑙𝐶𝑂3 × = 0.09901𝑔 𝐶𝑂3 1 𝑚𝑜𝑙 𝐶𝑂3 0.09901𝑔 𝐶𝑂3 × 100% = 41.83% 2.3672𝑔/10 Conclusion: In the unknown sample there was found to be a mass percent of 42.32% of bicarbonate and a mass percent of 39.04% carbonate. These were found using indirect titrations. Any error that could have occurred in the experiment would of come from the titrations themselves, or the standardizations. The standard deviation was acceptable being that it is not above 3%. Discussion: Primary standard is a compound that will react with and unknown concentration; the amount that it reacts has almost exact stoichiometric ratios and is able to accurately give the molarity of the unknown solution. Secondary standard is a solution that has a known concentration due to the use of a primary standard. Thus can be used to determine other concentrations. Titrant is the solution being used to titrate an analyte; it has a known concentration. Indirect titration is a titration where the analyte doesn’t react with the titrant. The reaction takes place with another analyte, which is directly related to what one is analyzing, thus is one is able to find the concentration indirectly. Sometimes they are called “back titrations” this is because the amount that reacts with the analyte must be known as well as the amount that doesn’t react with the analyte.