4.buffersandph - cxc chemistry
advertisement

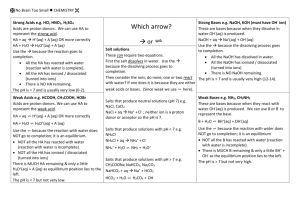
Kinetics & Equlibria 4. BUFFERS AND pH Buffers are solutions which have the ability to resist changes of acidity or alkalinity, their pHs remaining almost constant when small amounts of acid or alkali are added. One example of a system involving a buffer is blood, when pH is buffered at approximately 7.4. A small change of its pH of as little as 0.5 units is likely to be fatal. Acid buffers keep the pH below 7. Alkaline buffers keep the pH above 7. Acid Buffer Solutions Weak acid – ethanoic acid, CH3COOH Salt of acid – sodium ethanoate, CH3COONa Partial dissociation into ions CH3COO-(aq) + H (aq)+ CH3COOH (aq) (A) Complete dissociation units ions CH3 COO- (aq) + Na+ OH3 COO Na (aq) (B) The CH3COO- ions are present in majority and according to Le Chatelier’s Principle Equilibrium (A) are forced over to the left by the ethanoate ions from the sodium ethanoate. Adding OH- (Alkali) OH-(aq) + H+(aq) H2O(l) The OH-(aq) will remove the H+(aq) ions and shift equilibrium A to the right. This sluft will regenerate more hydrogen to react with further hydroxide. The hydroxide (OH-) is therefore “mopped up” by the hydrogen ions. In this way the pH does not change. Adding H+ (Acid) This time the buffer has to mop up the extra hydrogen ions from the acid. Equilibrium (A) shifts to the left, removing the majority of the added hydrogen ions by the ethanoate ions keeping the pH approximately constant. CH3COO + H+ CH3 COOH Alkaline Buffer solution Weak Alkali – Ammonium Hydroxide. NH3OH Salt of Alkali – Ammonium Chloride, NH3Cl Partial dissociation into ions NH3 (aq) + H2O (l) NH4+(aq) + OH- (aq) Ion solution, most of NH3 is solvated by water only a few reacts with water. Complete dissociation Copyright © Pooran Appadu 1 Kinetics & Equlibria NH4 + (aq) + Cl- (aq) NH4Cl (aq) Adding H+ (Acid) Adding hydrogen ions will be mopped up by hydroxide ion shifting equilibrium (c) to the right causing the pH to remain constant. Adding OH- (Alkali) Hydroxide ions are mopped up by ammonium ions NH (aq)+ + OH- NA4+(aq) Again the pH will remain almost constant. Calculating the pH of a buffer solution For a weak acid HA and its salt MA H+ + HA M+ MA A- + A- The expression for Kc of HA Kc = [H+] [A-] [HA] [HA] H+ = Kc [A-] Problem A buffer solution was made by adding 3.28g of sodium ethanoate to 1dm3 of 0.01 mol dn-3 ethanoic acid. What is the pH of this buffer? Ka (CH3COOH) = 1.7 * 10-5 mol dm-3 Solution [H+] [CH3COO-] Ka = [CH3COOH] [CH3COOH] H+ = Ka [CH3COO-] Molecular Mass = 82 Copyright © Pooran Appadu 2 Kinetics & Equlibria CH3COONa 3.82 No. of moles in 1 dm3 = 82 0.04 mol dm-3 = 0.01 H+ = 1.7 * 10-5 * 0.04 = 4.25 * 10-6 mol dm-3 pH = -log [H+] = -log [4.25 * 10-6] = 5.37 What happens to this pH if 1 cm3 of 0.1 mol dm-3 NaOH is added? Solution NaOH + CH3COOH CH3COONa + H2O i.e., in the buffer solution mentioned in the problem [CH3COOH] falls and [CH3COONa] rise 1 cm3 1.0 mol dm-3 NaOH = 0.001 mol dm-3 So after adding 0.001 mol dm-3 OH[CH3COOH] = 0.010 - 0.001 = 0.009 [CH3COONa] = 0.040 - 0.001 = 0.041 Acid [H+] = Ka Salt 0.009 = 1.7 * 10 -3 * 0.041 [H+] = 3.73 * 10-6 pH = 5.43 Therefore pH of the buffer changes by 0.06 units. 5.43 - 5.37 = 0.06 Units Copyright © Pooran Appadu 3 Kinetics & Equlibria Problem What will be the pH of a buffer solution made from a mixture of 0.1 mol dn-3 aqueous ammonia and 0.1 mol dm-3 ammonium chloride? In the formula below [Acid] pH = pKa –log [Base] What is the acid and what is the base? What is the value of the logarithm term? Given that pKb for ammonia is 4.75 what’s the pK2? What is the pH of the mixture? Solution NH4+ is the acid (proton donor), NH3 is base (proton acceptor) Zero; the ratio is 1 and log (1) = 0 Ratio 0.1: 0.1 = 1:1 = 1 pKa + pKb = 14 pKb = 4.5 pKa = 14.0 - 4.75 = 9.25 [0.1] pH = 9.25 – log [0.1] = 9.25 Buffers in Biochemical Environments Buffers keep the cell pH as constant as possible. Many tissues contain hydrogen phosphate ions, and since phosphoric acid is a weak acid, the equilibrium set up is: H3PO4- (aq) H+ (aq) + HPO42- (aq) When the [H+ (aq)] increases, the equilibrium is displaced from right to left, thus removing hydrogen ions from solutions. If the hydrogen in concentration decreases, the H3PO4- ions dissociate to release H+ ions. Hence, the buffer is maintained. Blood contains carbonate and hydrogen carbonate ions in equilibrium HCO3- (aq) CO32- (aq) + H+ (aq) The system acts as a buffer by regularizing the hydrogen ion concentration either to the left or to the right. If OH- accumulates in the tissues (and this is very dangerous) HCO3- ions absorb them forming CO32- ions. Copyright © Pooran Appadu 4 Kinetics & Equlibria HCO3-(aq) OH-(aq) CO32-(aq) + H2O (l) Amino acids have the formula H H Amide Group N H C R O C OH R – Alkyl or other group. As amino acids have both acidic and alkaline groups making them up, they can ionize as weak acids and as weak bases. Both the weakly acidic carboxyl group (-COOH) and the weakly basic amino group (-NH2) can act as buffers. H2NCHRCOOH (aq) + H2O (l) H3N+CHRCOOH (aq) + OH- (aq) H2NCHRCOOH (aq) + H2O (l) H2NCHRCOO- (aq) + H3O+ (aq) Proteins are formed by condensational polymerization. Amino acids peptides and proteins all help as important buffering agents in the bio-chemical environment. In summary cell contents are buffered to provide a suitable pH by a) Phosphates b) Hydrogen carbonate c) Amino acids, peptides and protein All chemical reactions are enzyme dependent and enzymes can only function at a pH range of two units (2 – 3 pH units) say pH 7. Buffers are also important in industries e.g. medicines and cosmetics. Copyright © Pooran Appadu 5