Buffers - slider-chemistry-12
advertisement

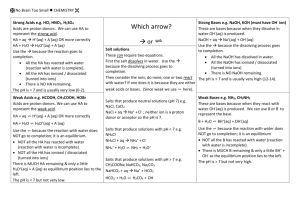
Buffers Year 12 Chemistry What is a buffer? • A buffer is a solution that resists changes in pH when small amounts of acid or base are added to it • There are 2 types: ▫ Acidic ▫ Alkaline Acidic buffers An acidic buffer has a pH less than 7 It is made from a equal molar concentrations of a weak acid and it’s conjugate base Example: ethanoic acid and ethanoate ion CH3COOH CH3COO- + H+ (weak acid) (conj. base) Acidic buffers Take a 0.1M solution of ethanoic acid: CH3COOH At equil: (0.09583M) What’s the pH of this solution? CH3COO- + H+ (0.00417M) (0.00417M) -log [H+] = 2.38 Now, say you add HCl to this equilibrium. The acetate ion is the only species available to reduce the added H+ and these are very low in concentration, so the pH will drop dramatically. What can you do to reduce this change? Add acetate ions (sodium acetate) Acidic buffers – adding acid CH3COOH - CH3COO + H + If we buffer the solution by adding 1M sodium acetate, what will happen? This will have the following effect: 1. Increase the amount of acetate ions, shifting the equilibrium to the left 2. Increase the pH to 4.76 3. When adding H+ ions, the extra acetate ions will react to reduce the [H+] 4. The solution resists changes to pH, meaning it is buffered Acidic buffers – adding base Add OH - CH3COOH - CH3COO + + H Adding base results in more OH- being added to the equilibrium. This extra amount of ions is removed by 2 processes. What are these? 1. 2. CH3COOH + OH- CH3COO- + H2O (ethanoic acid is removed by reaction with undissociated ethanoic acid. The small amount of H+ ions in the above reaction will also remove OH- ions, shifting the equilibrium to the right to make more H+ ions that further remove OH-. pH of buffers depends on concentration of conjugate pair pH vol. of 0.1M acetic acid (ml) vol. of 0.1M sodium acetate (ml) 3 982.3 17.7 4 847.0 153.0 5 357.0 653.0 6 52.2 947.8 Note: you can also get various pH buffers by changing the acid/base pair. Alkaline buffers An alkaline buffer has a pH greater than 7 It is made from a equal molar concentrations of a weak base and it’s conjugate acid Example: ammonia and ammonium ion + NH3 + H2O NH4 + OH (weak base) (conj. acid) - Alkaline buffers + - NH3 + H2O NH4 + OH (weak base) (conj. acid) Due to the weak nature of ammonia, this equilibrium will be well to the left. We can create a buffered solution by adding ammonium chloride. What will this do to the equilibrium? Addition of ammonium ions will shift the equilibrium even further to the left. The pH of this solution would be 9.25 for a 1M solution. Alkaline buffers – adding acid + - NH3 + H2O NH4 + OH What will happen if you add acid to this solution? Two processes: 1. NH3 + H+ NH4+ (removal by reaction with ammonia to produce more ammonium ion) 2. NH3 + H2O NH4+ + OH- (removal by reaction with OHto produce water Combines with H+ to form water Alkaline buffers – adding base + NH3 + H2O NH4 + OH - What will happen to the equilibrium if you add base? Adding base effectively adds OH-. This means: The ammonium ion reacts with the OH- to shift the equilibrium to the left, consuming most of the OH- ions. NH4+ + OH- NH3 + H2O Summary • Buffer solutions resist changes in pH when acids and alkalis are added • Buffers generally contain: ▫ Sufficient concentrations of a weak acid and it’s conjugate base OR weak base and it’s conjugate acid • The pH of buffer solutions depend on the concentrations and type of conjugate acid/base pairs that are used. Buffer applications Blood The pH of human blood must be maintained at 7.4 or serious health consequences can result. The buffering system that maintains this pH is H2CO3/HCO3-: H2CO3 H+ + HCO3- If blood increases in acidity, the additional H+ ions will react with the bicarbonate ions If blood increases in alkalinity, the H+ ions in the equilibrium will react and shift to the right Other buffers in the blood also help to maintain the required pH. Haemoglobin is itself a weak base that helps in this process Buffer applications Body cells The phosphate buffer system operates in the internal fluid of all cells. This buffer system consists of dihydrogen phosphate ions (H2PO4-) as hydrogenion donor (acid) and hydrogen phosphate ions (HPO42-) as hydrogen-ion acceptor (base). These two ions are in equilibrium with each other as indicated by the chemical equation below. H2PO4-(aq) H+(aq) + HPO42-(aq) Buffer applications Swimming pools The H2CO3/HCO3- buffer system that is used in the blood is also used in swimming pools. Sodium hydrogen carbonate is often added to swimming pools if it is proving difficult to maintain the pH between 7.2 – 7.4. The measurement “total alkalinity” in pools is a measure of OH- and HCO3-. This is effectively a measure of the buffering capacity of the water. If it is too low, then bicarbonate must be added.