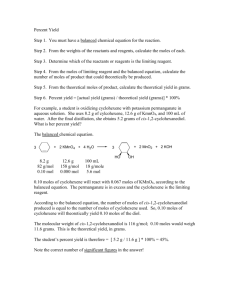
Review for chapter 9 exam

#1 Difficulty: 1
Define the term limiting reactant.
#2 Difficulty: 1
Define the term excess reactant.
#3 Difficulty: 1
Write the equation for percent yield.
#4 Difficulty: 1
The __________ yield is what is actually obtained through an experiment.
#5 Difficulty: 1
The ___________ yield is the maximum amount of product that can be produced from a given amount of reactant.
#6 Difficulty: 1
Write the mole ratio between potassium and potassium chloride.
____K + ____Cl
2
____KCl
#7 Difficulty: 1
Write the mole ratio between phosphoric acid and water.
____H
3
PO
4
+ ____NaOH ____Na
3
PO
4
+ ___H
2
O
#8 Difficulty: 2
If 3.5 moles of sodium react with excess oxygen, how many moles of sodium oxide are formed?
Balanced reaction: 4 Na + O
2
2 Na
2
O
#9 Difficulty: 2
If 9.33 moles of phosphoric acid reacts with excess sodium hydroxide, how many moles of sodium phosphate are formed?
Balanced reaction: 1 H
3
PO
4
+ 3 NaOH 1 Na
3
PO
4
+ 3 H
2
O
#10 Difficulty: 2
How many moles of oxygen are required to completely react 11.0 moles of carbon tetrahydride?
Balanced reaction:
#11
CH
4
Difficulty: 2
+ 2 O
2
CO
2
+ 2 H
2
O
How many moles of C
8
H
18
are required to produce 6 moles of carbon dioxide?
Balanced reaction: 2 C
8
H
18
+ 25 O
2
16 CO
2
+ 18 H
2
O
#12 Difficulty: 3
How many grams of sodium hydroxide are required to completely react 7.1 moles of lithum?
Balanced reaction: NaOH + Li LiOH + Na
#13 Difficulty: 3
How many grams of oxygen are required to completely react 6.5 moles of C
8
H
18
?
Balanced reaction: 2 C
8
H
18
+ 25 O
2
16 CO
2
+ 18 H
2
O
#14 Difficulty: 3
How many grams of oxygen are required to produce 5.66 moles of carbon dioxide?
Balanced reaction: CH
4
+ 2 O
2
CO
2
+ 2 H
2
O
#15 Difficulty: 3
How many grams of sodium hydroxide are required to completely react 14.5 moles of phosphoric acid?
Balanced reaction: 1 H
3
PO
4
+ 3 NaOH 1 Na
3
PO
4
+ 3 H
2
O
#16 Difficulty: 3
How many moles of sodium hydroxide are required to completely react 45.7 grams of phosphoric acid?
Balanced reaction: 1 H
3
PO
4
+ 3 NaOH 1 Na
3
PO
4
+ 3 H
2
O
#17 Difficulty: 3
How many moles of potassium chloride are produced when 55.6 grams of chlorine reacts with excess potassium?
Balanced reaction: 2 K + 1 Cl
2
2 KCl
#18 Difficulty: 3
How many moles of sulfuric acid are needed to completely react 78.4 grams of aluminum?
Balanced reaction: 2 Al + 3 H
2
SO
4
Al
2
(SO
4
)
3
+ 3 H
2
#19 Difficulty: 3
How many moles of tin (IV) oxide are necessary to completely react 295 grams of carbon?
Balanced reaction: 1 SnO
2
+ 2 C 1 Sn + 2 CO
#20 Difficulty: 3
How many moles of carbon monoxide are produced when 1044 grams of SnO
2
reacts with excess carbon?
Balanced reaction: 1 SnO
2
+ 2 C 1 Sn + 2 CO
#21 Difficulty: 4
How many grams of oxygen are necessary to produce 534 grams of sulfuric acid?
Balanced reaction: 2 SO
2
+ 1 O
2
+ 2 H
2
O 2 H
2
SO
4
#22 Difficulty: 4
How many grams of barium were reacted to produce 178 grams of hydrogen gas?
Balanced reaction: 1 Ba + 2 H
2
O 1 Ba(OH)
2
+ 1 H
2
#23 Difficulty: 4
How many grams of carbon tetrahydride are required to produce 98.4 grams of hydrochloric acid?
Balanced reaction: 1 CH
4
+ 4 Cl
2
1 CCl
4
+ 4 HCl
#24 Difficulty: 4
How many grams of chlorine are required to react with carbon tetrachloride to produce 101 grams of CCl
4
?
Balanced reaction: 1 CH
4
+ 4 Cl
2
1 CCl
4
+ 4 HCl
#25 Difficulty: 4
Determine the theoretical yield (in grams) of sodium chloride when 80.5 grams reacts sodium with excess chlorine.
Balanced reaction: 2 Na + Cl
2
2 NaCl
#26 Difficulty: 5
Determine the limiting reactant when 15 moles of sodium reacts with 15 moles of chlorine.
Balanced reaction: 2 Na + Cl
2
2 NaCl
#27 Difficulty: 5
Determine the limiting reactant when 13 grams reacts with 4 moles of chlorine.
Balanced reaction: 2 Na + Cl
2
2 NaCl
#28 Difficulty: 5
Determine the excess reactant when 66 grams of HCl react with 74 grams of potassium.
Balanced reaction: 2 HCl + 2 K 2 KCl + 1 H
2
#29 Difficulty: 5
Determine the limiting reactant when 95.5 grams of carbon tetrahydride react with 77 grams of oxygen.
Balanced reaction: CH
4
+ 2 O
2
CO
2
+ 2 H
2
O
#30 Difficulty: 3
What is the efficiency of a chemical reaction when 80 grams was recovered but 95 grams was expected?
#31 Difficulty: 3
Determine the actual yield of a reaction when 45 moles was expected and only 85% was recovered.
#32 Difficulty: 3
Determine the percent yield when 89.5 grams was recovered and 125 grams was expected.
#33 Difficulty: 6
If this reaction has a percent yield of 88%, how many grams of dinitrogen monoxide can be obtained from 50 grams of ammonium nitrate? (Hint: Calculate the theoretical yield (in grams) using stoichiometry. Then use the percent yield equation.)
Balanced reaction: 1 NH
4
NO
3
1 N
2
O + 2 H
2
O
#34 Difficulty: 6
If a reaction has a percent yield of 93%, how many grams of tin (II) fluoride can be obtained from 12 moles of tin? (Hint:
Calculate the theoretical yield (in grams) using stoichiometry. Then use the percent yield equation.)
Balanced reaction: 1 Sn + 2 HF 1 SnF
2
+ 1 H
2