Stoichiometry & Percent Yield Chemistry Problems
advertisement

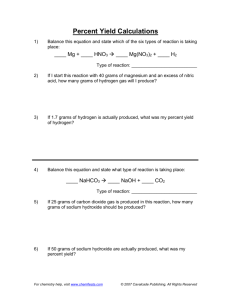
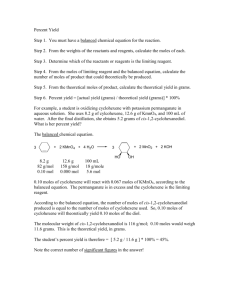
Stoich and % Yield Problems 1. How many grams of chlorine gas are needed in the production of 200.0 grams of bromine gas from sodium bromide? Cl2 + 2NaBr ® 2NaCl + Br2 2. Calculate the percent yield of a reaction that consumes 900.0 grams of potassium chlorate in the preparation of 90.0 grams of oxygen? 2KClO3 ® 2KClO2 + O2 3. In the reaction of 84.0 g of copper (II) oxide with hydrogen gas in a reaction with a 92.4% yield, Determine the mass of copper produced? CuO + H 2 ® Cu + H 2O 4. How many grams of sodium hydroxide will react with 1.50 x 10-3 g of phosphoric acid? 3NaOH + H 3PO4 ® 3H 2O + Na3PO4 5. If 320.0 grams of sodium carbonate react with calcium hydroxide in a 74.67% efficient reaction, how many grams of sodium hydroxide are formed? Na2CO3 + Ca(OH )2 ® 2NaOH + CaCO3 6. How many grams of sodium iodide are produced by the decomposition of 60.0 g of sodium iodate if the reaction is 89.4% effective? _ 2 _ NaIO3 ® _ 2 _ NaI + _ 3_O2 7. If 10.0 g of aluminum sulfide are produced by the 94.2% effective reaction of aluminum and sulfur, how many grams of sulfur were needed? _ 2 _ Al + _ 3_ S ® ___ Al2 S3 8. If 2.50 g of cupric sulfide is produced by decomposing cupric sulfate upon heating, how many grams of cupric sulfate was necessary if the reaction is 90.00% efficient? ___CuSO4 ® ___ CuS + _ 2 _O2 9. 125.0 g of FeS react to form 167.3 g of FeCl2: a. Determine the % yield of the reaction. b. Calculate, based on that percent yield, the moles of H2S that will be produced. FeS(s) + 2HCl(aq) ® FeCl2(aq) + H 2S(g) 10. If 1.487 moles of lithium oxide react with water through a 79.4% effective reaction, determine the mass of lithium hydroxide produced. Li2O(s) + H 2O(l) ® 2LiOH(aq) 11. In order to produce 100.0 g of NaCl based on the following 84.3 % efficient reaction, determine the moles of each reactant required. Na 2SO4(aq) + CaCl2(aq) ® CaSO4(s) + 2NaCl(aq) 12. 0.941 moles of calcium hydroxide react to form 30.55 g of water. Determine the percent yield of the reaction. Ca(OH)2(aq) + 2HCl(aq) ® CaCl2(aq) + 2H 2O(l) 13. What mass of ethane must react in order to produce 4.73 moles of carbon dioxide if the following reaction has a 91.8 % yield? 2C2H 6 + 7O 2 ® 4CO 2 + 6H 2O 14. 4.84 moles of oxygen react in the following 87.9 % yield reaction. Calculate the mass of each product produced. 2Cu2S + 3O 2 ® 2Cu2O + 2SO2