Ch. 12 Study Guide
advertisement

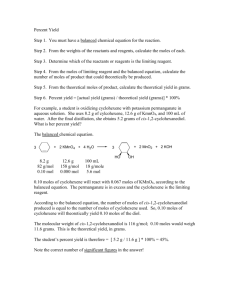
Chapter 12 Study Guide - Stoichiometry TEST: Thursday 3/10/11 12.1 The Arithmetic of Equations 1. Define stoichiometry 2. What is always conserved in every chemical reaction? 12.2 Chemical Calculations 1. Define mole ratio 2. In a balanced chemical equation, where can one find the numbers to make up the mole ratio? 3. For the following reaction: 2H2S (g) + 3O2 (g) 2SO2 (g) + 2H2O (g) a. What is the mole ratio of hydrogen sulfide to water? b. What is the mole ratio of oxygen to sulfur dioxide c. What is the mole ratio of water to oxygen? 1 Steps to solve Mole-Mole problems: 1. Write down the given 2. Multiply the given by the mole ratio using the coefficients in the balanced equation (numerator = unknown, denominator = known) Mole-Mole Practice: 1. How many moles of hydrogen gas are needed to react completely with two moles of nitrogen gas? 3H2 + N2 2NH3 2. How much ammonia (NH3) is produced from two moles of nitrogen gas? 3H2 + N2 2NH3 3. How many moles of oxygen gas are produced from the decomposition of six moles of potassium chloride? 2KClO3 2KCl + 3O2 4. How many moles of potassium chloride are produced from the decomposition of six moles of potassium chlorate? 2KClO3 2KCl + 3O2 5. How many moles of hydrogen gas are produced from the reaction of three moles of zinc and an excess of hydrochloric acid? Zn + 2HCl ZnCl2 + H2 2 Steps to solve Mass-Mass problems: 1. Write down the given 2. Change the mass of the given to moles of the given (use the molar mass) 3. Change the moles of given to moles of unknown (use mole ratio) 4. Change the moles of unknown to mass of unknown (use molar mass) Mass-Mass Practice: 1. How many grams of potassium chloride are produced if 25g of potassium chlorate decompose? 2KClO3 2KCl + 3O2 2. For the equation in #1, how many grams of oxygen are produced if 25g of potassium chlorate decompose? 3. How many grams of silver chloride are produced from 5.0g of silver nitrate reacting with an excess of barium chloride? 2AgNO3 + BaCl2 Ba(NO3)2 + 2AgCl 4. For the equation in #3, how many grams of barium nitrate are produced from 5.0g of silver nitrate? 3 THE MOLE ROADMAP – use it for all your stoichiometry problems!!! Mixed Problems: 1. Ten grams of calcium carbonate was produced when carbon dioxide was added to lime water (calcium hydroxide in solution). What volume of carbon dioxide at STP was needed? [ CO2(g) + Ca(OH)2 (aq) → CaCO3 + H2O ] 2. When 11.2 liters of hydrogen gas is made by adding zinc to sulfuric acid, what mass of zinc is needed? [ Zn + H2SO4 → H2 + ZnSO4 ] 3. How many grams of carbonic acid is produced when 55 liters of carbon dioxide is pressed into water? [ CO2 + H2O → H2CO3 ] 4 4. How much strontium bromide is needed to add to chlorine gas to produce 75 liters of bromine? [ SrBr2 + Cl2 SrCl2 + Br2 ] 5. What mass of ammonium chlorate is needed to decompose to give off 200 liters of oxygen? [ 2NH4ClO3 3O2 + 2NH4Cl ] 6. How many grams of sodium do you have to put into water to make 30 liters of hydrogen at STP? [Na + H2O → NaOH + H2 ] 7. Ammonia gas (NH3) and hydrogen chloride gas (HCl) combine to make ammonium chloride (NH4Cl). What volume of ammonia at STP is needed to react with 47.7 liters of hydrogen chloride at STP? ANSWERS TO MIXED PROBLEMS #1-7 1. 2.24 L 2. 32.7 g 3. 152 g 5. 604 g 6. 61.6 g 7. 47.7 L 5 4. 828 g 12.3 Limiting Reagent and Percent Yield: 1. Define theoretical yield. How is it calculated? 2. Define actual yield 3. What are some reasons for losses that account for the difference between theoretical and actual yield? Steps to find theoretical yield: 1. Write out the balanced equation 2. Calculate the mass of the unknown – this is the theoretical yield. Formula to find percent yield: percent yield = actual yield x 100% theoretical yield Percent Yield Practice Problems: 1. When 5.00 g of KClO3 is heated it decomposes according to the equation: 2KClO3 2KCl + 3O2 a) Calculate the theoretical yield of oxygen. b) Give the % yield if 1.78 g of O2 is produced. 2. 138 g H2O is produced from 16 g H2 and excess O2 according to the equation: 2H2 + O2 2H2O a) What is the theoretical yield of water? b) Calculate the % yield of water. 6 3. What is the % yield of NH3 if 40.5 g NH3 is produced from 20.0 mol H2 and excess N2? a) Write the balanced chemical equation b) What is the theoretical yield of NH3? c) Calculate the % yield of NH3. 4. 107 g of oxygen is produced by heating 300 grams of potassium chlorate for the following reaction: 2KClO3 2KCI + 3O2 a) What is the theoretical yield of oxygen? b) Calculate the % yield of O2 5. What is % yield of ferrous sulfide if 3 mol Fe produces 220 grams of ferrous sulfide? Fe + S FeS a) What is the theoretical yield of ferrous sulfide? b) Calculate the % yield of ferrous sulfide ANSWERS TO % YIELD PROBLEMS: 1a) 1.9g O2 1b) 93.7% O2 2a) 143g H2O 2b) 96.7% H2O 3a) N2 + 3H2 2NH3 3b) 227g NH3 3c) 17.8% NH3 4a) 117.5g O2 4b) 91.1% O2 5a) 263.7g FeS 5b) 83.4% 7 4. Define limiting reagent 5. Define excess reagent Steps to determine the limiting & excess reagents 1. Calculate the yield (mass of product) using each of the reactants (ie. calculate the yield twice – once for each reactant) 2. The yield that is the smallest amount is the theoretical yield the reactant used for this calculation is the limiting reagent the other reactant is the excess reagent Limiting Reagent Practice Problems: 1. When copper (II) chloride reacts with sodium nitrate, copper (II) nitrate and sodium chloride are formed: CuCl2 + 2 NaNO3 Cu(NO3)2 + 2 NaCl a) If 15 grams of copper (II) chloride react with 20 grams of sodium nitrate, how much sodium chloride can be formed? (Hint: you must do 2 calculations) b) What is the limiting reagent for the reaction? 2. A 2.00 g sample of ammonia is mixed with 4.00 g of oxygen: 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) a) How much NO can be formed? b) Which is the limiting reagent? 8 3. For the reaction: K + O2 K2O, a) how many grams of K2O will be produced from 0.50 g of K and 0.10 g of O2? b) Which is the limiting reagent? 4. For the reaction: Na2O + H2O NaOH a) How much NaOH could be made from 12.4 g of Na2O and 42.1 g of H2O? b) What is the limiting reagent? 5. For the reaction: C + H2 CH4 a) How much CH4 will be made when 10.0 g of H2 reacts with 5.0 g of C? b) Which is the limiting reagent? ANSWERS TO LIMITING REAGENT PRACTICE PROBLEMS: 1a) 15g CuCl2 yields 12.9g NaCl 20g NaNO3 yields 13.6g NaCl 1b) Since 12.9g NaCl is the smaller amount, CuCl2 is the limiting reagent. 2a) 2g NH3 yields 3.53g NO 4g O2 yields 3.00g NO 2b) Since 3.00g NO is the smaller amount, O2 is the limiting reagent. 3a) 0.50g K yields 0.60g K2O 0.10g O2 yields 0.59g K2O 3b) Since 0.59g K2O is the smaller amount, O2 is the limiting reagent. 4a) 12.4g Na2O yields 16g NaOH 42.1g H2O yields 187g NaOH 4b) Since 16g NaOH is the smaller amount, Na2O is the limiting reagent. 5a) 10g H2 yields 40g CH4 5.0g C yields 6.7g CH4 5b) Since 6.7g CH4 is the smaller amount, C is the limiting reagent. 9