Supplemental Material_Morphology and Chemical
advertisement

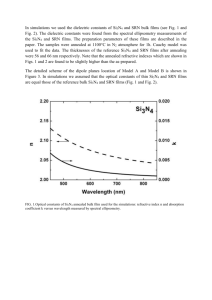
Supplementary information for Morphology and Chemical Termination of HF-Etched Si3N4 Surfaces Li-Hong Liu,1 William J. I. Debenedetti,1 Tatiana Peixoto,1 Sumeyra Gokalp,1 Natis Shafiq,1 Jean-François Veyan,1 David J. Michalak,2 Rami Hourani,2 Yves J. Chabal1,a 1 Department of Materials Science and Engineering, University of Texas at Dallas, Richardson, Texas 75080, USA 2 a Components Research, Intel Corporation, Hillsboro, Oregon 97124, USA Author to whom correspondence should be addressed. Electronic mail: chabal@utdallas.edu I. Si3N4 refractive index measurements: The data were fit on a variable angle spectroscopic ellipsometer from 600-1000 nm wavelength using 60 and 75 degree incidence. The psi and delta curves were fit to an optical model using Si, 1.5 nm SiO2 (native oxide), and a Cauchy model floating thickness, A, and B for the Si3N4 layer. Refractive index at any wavelength (–in units of microns) can be calculated by using the formula: Refractive index () = A + B/(2) Refractive index at 633 nm = 1.9956, Error = 0.006808 Refractive index at 637 nm = 1.9917, Error = 0.006756 1 II. Etching of nitride films and removal of byproducts from the surface (IRAS experiments) Fig S1. Infrared absorption spectra of silicon nitride surface etched by 1 % (0.32 M) HF(aq) for 1 min. The negative absorption bands at 1056 cm-1 and 1200 cm-1 are associated with SiO2 TO and LO phonon modes, indicating removal of some of native SiO2 or oxynitride on the initial Si3N4 surface. In adition, the removal of some Si3N4 films by 1 % (0.32 M) HF treatment is also evidenced by the broad and structured negative absorption bands at 827 cm-1 and 1134 cm-1, corresponding to the Si3N4 TO and LO phonon modes. Fig S2. Infrared absorption spectra of four silicon nitride surfaces etched by 10 % (3.18 M) HF(aq) for 1, 2, 3 and 4 min each, followed by 10 s water rinses. These short-time rinses (10 sec) are sufficient to remove surface species produced by short HF etching times (1-3 min) because smaller salt crystals are formed for short HF etching times. However, for longer HF etching times (4 min or longer), 10 s DI water rinse is not sufficient to fully remove the Si3N4 etching byproduct salts formed and/or deposited on the surface. While the microcrystallites are sparse, occupying ~1-10% of the surface area (the structures shown in Figs. 2 and 3 are typical but there are many areas with much higher density), they are thick ~100 nm or about 50 monolayers). So 2 the equivalent coverage of the surface is somewhat greater than one monolayer on average. In addition, the dynamic dipole moment of the salt crystal modes is very strong, stronger than that of adsorbed NH x, leading to ~ 2 times larger absorption intensity per hydrogen. Altogether, the observed band intensities in the IR spectra are consistent with the presence of these microcrystals. III. Detection of surface NHx species (1550 cm-1 band studied by IRAS) Fig S3. Infrared absorption spectra of silicon nitride surface etched by 10 % (3.18 M) HF(aq) for 4 min. Inset: Surface NHx species are barely detectable at 1550 cm-1 with an intensity ~5×10-5. FTIR RMS noise (1700-1600 cm1 ): 4.8×10-6. Estimates of the NHx concentration are difficult. Current studies of aldehyde functionalization (unpublished) indicate that, for the current level of detected NHx modes, about 10% of the surface is covered. [to be published] IV. F and O Surface coverage estimated by XPS, with controlled sputtering of surface species (Gas Cluster Ion Beam sputtering experiment) The thickness of the overlayer formed by F, C and O on the Si3N4 substrate can be estimated by measuring the attenuation of the substrate elements, Si and N, by that overlayer, compared to the intensities obtained on a clean substrate (i.e. without F, C and O), using the standard equation: 𝐼𝑜𝑣 𝐼𝑆𝑢𝑏 = 𝐼𝑜𝑣0 [1−𝑒 −𝑑⁄𝜆𝑜𝑣 sin 𝜃 ] 𝐼𝑆𝑢𝑏0 𝑒 −𝑑⁄𝜆𝑆𝑢𝑏 sin 𝜃 (I) where ISub and ISub0 represent the measured areas of the substrate feature respectively with and without the overlayer; IOv is the measured area of the overlayer feature, IOv0 stands for the estimated area of an infinitely thick overlayer feature, λSub and λOv are the electron effective attenuation length respectively for the substrate electron kinetic energy and the overlayer electron kinetic energy (in the overlayer material), d the thickness of overlayer and the electron take off angle. This estimate requires therefore the measurement of the clean substrate, i.e. the selective removal of the overlayer without perturbation of the substrate element concentration. Right after the chemical etching, the samples are introduced into a Versa Probe II (Physical Electronics) XPS chamber at a base pressure of 4.8×10-8 Pa. This experimental chamber is equipped with two kinds of sputter guns: an Ar+ sputter gun generating a beam of Ar ion from 250 eV up to 5 KV, and a Gas Cluster Ion Beam (CGIB) 3 producing clusters from 1000 up to 2500 Ar atoms at kinetic energy from 5KV up to 25 KV. During sputtering, the pressure in the chamber was around 2×10-6 Pa. On the Si3N4 surface which etched by 10 % (3.18 M) HF for 4 min and rinse in DI water 30s, the C has been easily removed without modifying the substrate stoichiometry by sputtering the sample with clusters size 2500 at 5KV, i.e. 2 eV per Ar atom (see Table S1, line 2 and Fig S4 curves black and red). This clearly indicates the the C is not chemically bound to the surface but most likely physisorbed. This contamination forming an overlayer on top of the F and O overlayer, comes from the exposure of the sample to air during the transfer to the XPS chamber, and has no other effect rather than a general screening for all other species. During those sputtering cycles, the substrate stoichiometry remains unchanged as indicated in Table S1 (two last columns labeled Si3N4). To remove the F and O, a stronger cluster beam (size 2000 at 15KV, i.e. 7.5eV per Ar atom) is needed. Unfortunately, some N is also removed (preferentially over Si), and therefore the peak intensities for Si and N are no longer in a 3 to 4 ratio (See Table S1: last two columns labeled Si3N4). It is therefore not possible to give a good estimate of the fluorine concentration at the sample surface. Nevertheless, we can establish a relative surface concentration of 45 % F and 55% O in the overlayer for both species that are chemically bound to the surface. Fig S4. Comparison of X-ray photoelectron spectra of the C 1s, F 1s, O 1s before and after GCIB treatment. Si3N4 surface etched by 10 % (3.18 M) HF(aq) for 4 min and rinsed by DI water right away for 30 sec (black line), GCIB (2 eV/atom) treatment on etched surface for 2.5 min (red line), and GCIB (7.5 eV/atom) treatment on etched surface for 2.5 min (green line). For each sputtering, the sputtered area was 1×1 mm2. Table S1. Concentration of C, O, F, N and Si before, during and after the subsequent sputtering cycles based on the relative peak areas only and taking into account the respective sensitivity factors: C: 0.3027; O: 0.7478; F: 1.0562; N: 0.4942; Si: 0.3959. Before sputtering After Ar cluster 5kV After Ar cluster 15kV C O 1s F 1s ratio ratio ratio Area Area Area % % % 3 430 4.9 1520 3.6 1587 0.6 86 4.3 1330 3.5 1542 0 0 2.2 640 0 0 4 N 1s ratio Area % 52.4 10433 54.5 11411 51.4 9034 Si 2p Si3N4 ratio N Si Area % 57.14% 42.86% 36.1 5690 59.21 40.79 37.2 6083 59.43 40.57 46.2 6238 52.66 47.34