Finding Footholds in Laminitis Research: 2014
advertisement

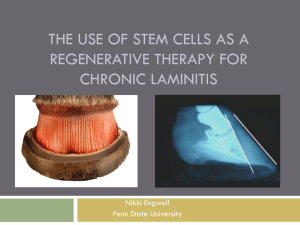
Equine Laminitis- gaining footholds in a complex disease: a review of pathophysiology, current experimental models, and future therapeutic interventions. Julie B. Engiles, VMD, DACVP. University of Pennsylvania, School of Veterinary Medicine, Department of Pathobiology, New Bolton Center, Kennett Square, Pennsylvania, USA. Laminitis is an important cause of lameness, morbidity, and mortality in horses, and is economically costly to the equine industry. A 1998 USDA survey of 1178 non-racetrack horse operations, including over 28,000 horses representing 28 states and approximately 83.9% of non-racehorses in the US, estimated laminitis to account for 7.5-15.7% of lameness problems with death or euthanasia resulting from laminitis in 4.7% of these horses.32 However, the estimated frequency of disease occurrence as reported in the literature varies from 1.5-34%, depending on underlying etiology or horse populations.64 A recently published cohort study of equine laminitis in Great Britain (2009-2011) estimates the prevalence of veterinary-diagnosed active laminitis to be 0.47% (approximately 1 in 200 horses seen by veterinarians). In comparison, a retrospective study performed at a tertiary care facility investigating the factors associated with non-survival in horses treated for laminitis from 1986-2003 reports 247 out of 591 horses hospitalized for laminitis died or were euthanized, and that the severity of lameness was the most significant prognostic indicator, not the presence of systemic inflammatory diseases (e.g. phlebitis/ thrombophlebitis, pneumonia, or treatment with NSAIDs).48 The equine foot is a highly specialized appendage that integrates several different anatomic elements including a keratinized epidermis, a highly vascularized dermis and corium having valveless veins, as well as other connective tissue elements such as the distal phalanx (DP) with its ligamentous entheses, the fibroadipose tissue of the heel bulbs and digital cushion, the collateral cartilages, and tendons (e.g. the insertion of the deep digital flexor tendon). [Figure 1] Although the pathogenesis of laminitis is incompletely understood, the target of the disease is the suspensory apparatus of the distal phalanx (SADP) that consists of extensively interdigitated epidermal and dermal lamellae that attach and suspend the DP within the hard keratinized hoof capsule through this inner portion of the hoof wall (stratum internum).53 The lamellae comprise 500-600 primary lamellae, each having 150-200 secondary lamellae that, via a basement membrane at the dermo-epidermal junction, provide an large surface area (~0.8m2) for attachment and suspension.54 [Figure 2] The blood supply to DP that originates from the palmar/plantar digital arteries continues via vascular canals that extend through its semi-porous dorsal cortex, to supply the overlying epidermal/dermal lamellae. The blood vessels supplying the primary and secondary lamellae contain valveless veins and numerous arteriovenous anastomoses that allow for shunting of blood within hoof wall for pressure distribution and thermoregulation.54 In fact, experimental intraosseous infusion of the DP via a cannulated bone screw demonstrated clinically safe and efficacious delivery of an aminoglycoside (Gentamicin) to lamellar tissues at much higher concentrations than detected in peripheral serum.45 Given the unique anatomical and functional properties of the SADP, it is not surprising that unique molecular components of lamellar epithelium and mesenchyme have also been discovered. Examples include the identification of novel keratin isoforms (K42 and K124) as the most abundant cytoskelatal proteins of lamellar keratinocytes, the production of large polysulfated glycosaminoglycans (aggrecan, versican, and hyaluronic acid: large water-binding molecules predominantly found in cartilage tissues under extensive compressive mechanical stress) localized within lamellar epidermal keratinocytes, and ubiquitous expression of proteoglycans that are detectable by oligosaccharide-binding plant lectins such as wheat germ agglutinin (WGA) throughout the extracellular matrix of dermal lamellae, as well as delineating intercellular membranes of epidermal keratinocytes.10, 11, 50 In addition to not containing resident neutrophils, normal lamellae demonstrate less superoxide dismutase activity, and also exist in a relative hypoxic state as compared to other equine tissues.19, 51 Histologic architectural variations within primary and secondary epidermal lamellae (PELs and SELs, respectively) exist depending on location within the hoof wall as well as age and athletic history of the horse, likely representing a spectrum of physiologic responses due to normal growth and remodeling of the hoof wall, as well as pathologic responses to prior (multi)focal injury. 24, 33 The pathogenesis of equine laminitis is complex, and to date, incompletely understood with no known cure. The disease predominantly affects the sensitive epidermal and dermal lamellae of the inner hoof wall that typically results in biomechanical failure of these tissues with eventual rotation and distal displacement (“sinking”) of the DP, often necessitating humane euthanasia because of intractable pain and dysfunction. Inadequate tissue healing and aberrant regenerative responses result in hyperproliferation of epidermal/dermal lamellae, and correspond to the formation of the lamellar wedge, the most characteristic radiographic and gross lesion of chronic laminitis.8, 12, 36 [Figure 3] The disease can be divided into stages depending on clinical presentation, with a developmental, or subclinical “eclipse” phase that precedes clinical signs manifesting as systemic illness (e.g. fever) or lameness (e.g. Obel Grade lameness stages 1-4), depending on the inciting cause. Using experimental induction models of laminitis, early “acute” histololgic changes within the first 48 hours post induction, coinciding with the initial onset of Obel Grade 1 lameness, include narrowing and lengthening of the SELs, loss of polarity and rounding of basal keratinocyte nuclei, and increases in basal keratinocyte necrosis and mitosis.17 Histologic changes corresponding to the subacute into chronic stages of experimental and natural (e.g. spontaneous) cases of laminitis include necrosis and fragmentation of SELs with basement membrane separation at the dermoepidermal junction, displacement of the keratinized axis with lengthening of PELs, dysplastic epidermal lamellar regeneration, and dermal inflammation.44, 46, 57 [Figure 4] The initial acute to subacute changes in the lamellae are typically not detectable by conventional radiology, but can be detected with more sensitive imaging modalities such as magnetic resonance imaging (MRI). 2 Biomechanical failure of the SADP corresponding to the characteristic distal displacement, or “sinking” of the distal phalanx often coincides with the proliferation of a markedly dysplastic, hyperkertotic regenerative reponse of the epidermal lamellae, corresponding to the lamellar wedge.13, 27 [Figure 5] In addition to structural alterations in the lamellae, laminitis results in structural alterations to other tissues including the digital microvasculature, digital nerves and spinal dorsal root ganglia, and the distal phalanx. More complete characterization of changes in these tissues are ongoing and, given their potential impact on tissue regeneration, disease progression, and important co-morbidities such as pain, are considered to have potentially important contributions to the pathogenesis.20, 31, 65 The development of spontaneous equine laminitis is associated with many different environmental exposures or clinical risk factors including: deranged endocrine/metabolic states (e.g. hyperinsulinemia and Equine Metabolic Syndrome-EMS, Pars Pituitary Intermedia Dysfunction- PPID), high carbohydrate diet (e.g. “pasture founder” or “grain overload”, systemic inflammatory and infectious diseases (e.g. Salmonellosis, Potomac Horse Fever, sepsis), vascular disease (e.g. local or generalized ischemic states, vasculitis/vasculopathy), repetitive local trauma (e.g. “road founder”), and toxic causes (e.g. Black Walnut Toxicity). 18, 49 Risk factors and clinical outcomes also differ dramatically among different populations of horses, such as in different breeds of horses, or hospitalized versus non-hospitalized horses.1, 48, 63 However in spite of these various and numerous risk factors, very few absolute “causes” of laminitis have been confirmed by experimental models, making research investigations of laminitis difficult.29 In the past, laminitis research largely depended on anecdotal or observational descriptive manuscripts; however to better characterize the pathogenesis, more recent studies have incorporated experimental induction models for investigating the morphological and molecular differences between normal versus laminitic feet. Current induction models include: 1) carbohydrate overload (CHO) model, considered to mimic sepsis-associated laminitis, induced by nasogastric administration of wood starch/gruel slurry or oligofructose; 2) hyperinsulinemic (HI) model, considered to mimic endocrinemetabolic-associated laminitis, induced by intravenous infusion of insulin and glucose to achieve a prolonged euglycemic/hyperinsulinemic “clamp”; and 3) Black Walnut Extract (BWE) model, considered to mimic Black Walnut toxin-associated laminitis, induced by nasogastric administration of an aqueous extract from Black Walnut heartwood shavings.6, 26, 37 Since naturally occurring laminitis is most frequently associated with risk factors and processes similar to the CHO and HI models, the focus of this review will pertain to these two experimental induction methods. Experimental introduction of excessive alimentary carbohydrate results in a microflora shift in the equine hindgut that reliably produces laminitis in most equine subjects.52 Recent studies that include microbiomic analyses of equine hindgut microflora show a post-induction shift to acid-producing Gram positive bacteria (e.g. Lactobacillus sp. and Streptococcus sp.) as well as potentially pathogenic Gram negative bacteria (e.g. Veillonella sp. and Serratia sp.), which are thought to contribute to the intestinal mucosal damage and the pro-inflammatory state that progresses to laminitis.42, 47 Transcriptomic, proteomic, and immunohistochemical evaluations of laminitis in horses induced by CHO reveal endotoxemia and a molecular profile consistent with a systemic as well as lamellar-specific inflammatory response including systemic platelet activation with release of serotonin and thromboxane, and dysregulation of fatty acid metabolism with significant depletions in citrulline (a potential biomarker of critically ill animals), lamellar inflammatory gene expression (IL-1, IL-6, IL12p35, COX2, E-selectin, ICAM-1, CXC chemokines and CC chemokines), and lamellar infiltration by leukocytes.3, 22, 23, 38, 56 In addition, CHO induced laminitis results in the production of catalytically active ADAMTS-4 with depletion of versican from basal keratinocytes, accompanied by regional basement separation of secondary epidermal lamellae.60 Further investigation showed that versican depletion is associated with suppression of the canonical Wnt signaling pathway that maintains the epithelial phenotype, as evidenced by concomitant depletion of -catenin and -integrin, and that an increased expression of MMP-13 collagenase in basal keratinocytes is accompanied by the loss of collagen I, Fibronectin, chondroitin and keratan sulfate glycosaminoglycans, and eosin-staining material within adjacent secondary dermal lamellae.61, 62 Evaluation of epidermal lamellar keratinocytes isolated by laser capture microdissection confirms production of inflammatory mediators and extracellular matrix regulators by basal keratinocytes, indicating that these cells are active participants in the molecular and cellular events leading to lamellar structural failure, and are not merely “innocent bystanders” as once thought.39 However, although the CHO induction model may resemble sepsis-associated laminitis in horses, a natural disease model of “pasture associated laminitis” produced by feeding a high nonstructural carbohydrate diet (hay chop supplemented with sweet feed and oligofructose) to lean and obese mixed breed ponies only resulted in increased COX-2 expression as quantitatively assessed by immunohistochemistry, not pro-inflammatory mediator expression or inflammatory infiltration of lamellar tissues, concluding that perhaps the cellular and molecular events occurring in the model of sepsis-associated laminitis may not be representative of pasture-associated laminitis.7 A different experimental induction technique thought to model endocrinopathic and pasture-associated laminitis is the hyperinsulinemia (HI) model that induces a hyperinsulemic state by prolonged intravenous infusion of insulin (6 mIU/kg bwt/min) with a variable rate intravenous infusion of 50% glucose to maintain a euglycemic state until the onset of Obel Grade 2 lameness (~48 hour period).37 Although all treated horses developed laminitis with degenerative and necrotic changes to the SELs similar to that described for the CHO model, in contrast to the CHO model, the inflammation with lamellae induced by HI was much less severe, and there was minimal evidence of matrix metalloproteinase upregulation.17, 55 Interestingly, a less marked hyperinsulemia induced in horses by intravenous infusion of glucose for a period of 48 hours did not result in lameness, and lamellar pathology was much less severe, suggesting that a glucose overload alone is unlikely to be the underlying mechanism of endocrinopathic and pasture-associated laminitis.16 In addition, the pro-inflammatory cytokine response observed in the CHO model, was not observed in either euglycemic or hyperglycemic HI models.14 Results from another study evaluating different isoforms of glucose transporters in lamellae, skeletal muscle, and heart from the HI model suggest that epidermal lamellar tissue may function independently of insulin and that insulin resistance may not be a necessary component of laminitis pathogenesis in this model, thus the pathogenesis of endocrinopathic laminitis remains elusive. 5 Although these experimental induction models have provided valuable insight into the molecular and anatomical events preceding structural failure of the lamellar interface, their direct translation to events occurring in naturally occurring laminitis is limited, thus the importance of investigating spontaneous cases of laminitis should not be overlooked. However, given the nature of clinical laminitis, and that biopsy of the hoof wall is considered relatively invasive and not routine, thorough pathological evaluation typically requires euthanasia, which often occurs after the devastating secondary effects due to complete biomechanical failure of the SADP, precluding of evaluation of pathogenically important yet clinically silent developmental through acute stages of laminitis. Perhaps for logistical reasons, investigations of spontaneous laminitis mostly comprise either case controlled or observational retrospective studies of clinical risk factors and survival outcomes, with concurrent clinical pathologic parameters.4, 5, 21, 30, 34, 43, 48, 59 Given the limited numbers of studies including anatomic pathologic investigations in conjunction with detailed historical and clinical pathologic data, a Laminitis Discovery Database has been developed at New Bolton Center to provide a comprehensive image, serum and tissue repository for the study of naturally occurring laminitis.25 This database includes samples from over 125 normal (N=55) and laminitic (N=70) horses having various associated risk factors (inflammatory-sepsis, support limb overload, endocrinopathic-metabolic) and in various stages of disease, including developmental and acute stages as well as subacute and chronic. Two to four front and hind feet are harvested from each horse, totaling 304 feet. The goal is to correlate transcriptomic and proteomic data with gross anatomic and histopathologic changes to more completely evaluate naturally occurring laminitis, and to potentially identify serum biomarkers for early disease detection and prevention, or novel targets for therapeutic interventions. In addition to abundant gross and histomorphometrical data, pathologic evaluation of these tissues has identified reduced expression of the epidermal stem cell marker p63 in lamellae from horses with chronic laminitis, and medullary activation with osteoclastic osteolysis of the distal phalanx in early-onset as well as chronic stages of laminitis.9, 20 The tissue repository has also provided lamellar epidermal cells to develop an epithelial stem cell-selective and 3-dimensional culture system as an in vitro organotypic model system for pathophysiologic studies of lamellar epithelial cell-cell interactions, therapeutic interventions, and epidermal regenerative medicine.40 However, in spite of intense efforts to identify novel therapeutic targets, to date very little has changed regarding treatment of laminitis. Distal limb cryotherapy (e.g. application of tall ice boots to the level of carpi or hocks) has been shown to reduce the severity of lamellar pathology in a CHO experimental induction model, and also reduce the incidence of laminitis in a clinical population of horses with colitis at risk for sepsisassociated laminitis.35, 58 Regarding dietary and exercise management, one casecontrolled study demonstrated that regular low intensity exercise did significantly decrease the concentration of two plasma inflammatory markers (serum amyloid AsAA, and haptoglobin- Hp) in both previously laminitic and non-laminitic ponies, and another in vitro study identified the -acid phytochemical from hops in beer (Humulus lupulus L.) prevented the growth of fructan-fermenting equine hindgut microflora.28, 41 Experimental investigations for gene therapy delivery to inhibit lamellar matrix metalloproteinase activation, and mechanically dynamic shoeing to prevent support-limb laminitis are on-going (personal communications, Dr. Dean Richardson). Given the complexity of experimental and naturally occurring models of laminitis, the discovery of a “magic bullet” treatment is unlikely; however continued dedication of resources to this area of research underscores its importance to the veterinary and equine community at large. Although good progress has been made since the application of molecular techniques to laminitis pathophysiologic research, many more critical investigations will be required before the step-wise molecular and anatomic progression to lamellar failure is fully elucidated for each associated risk factor, so that successful targeted therapeutic intervention or reliable disease prevention can occur. References 1. Alford P, Geller S, Richrdson B, et al. A multicenter, matched case-control study of risk factors for equine laminitis. Prev Vet Med. 2001;49(3-4):209-222. 2. Arble JB, Mattoon JS, Drost WT, et al. Magnetic resonance imaging of the initial active stage of equine laminitis at 4.7 T. Vet Radiol Ultrasound. 2009;50(1):3-12. 3. Bailey SR, Adair HS, Reinemeyer CR, et al. Plasma concentrations of endotoxin and platelet activation in the developmental stage of oligofructose-induced laminitis. Vet Immunol Immunopathol. 2009;129(34):167-173. 4. Bamford NJ, Potter SJ, Harris PA, et al. Breed differences in insulin sensitivity and insulinemic responses to oral glucose in horses and ponies of moderate body condition score. Domest Anim Endocrinol. 2014;47:101-107. 5. Bertin FR, Reising A, Slovis NM, et al. Clinical and clinicopathological factors associated with survival in 44 horses with equine neorickettsiosis (Potomac horse Fever). J Vet Intern Med. 2013;27(6):15281534. 6. Black SJ, Lunn DP, Yin C, et al. Leukocyte emigration in the early stages of laminitis. Vet Immunol Immunopathol. 2006;109(1):161-166. 7. Burns TA, Watts MR, Weber PS, et al. Laminar inflammatory events in lean and obese ponies subjected to high-carbohydrate feeding: implications for pasture-associated laminitis. Equine Vet J. 2014. 8. Carter RA, Engiles JB, Megee SO, et al. Decreased expression of p63, a regulator of epidermal stem cells, in the chronic laminitic equine hoof. Equine Vet J. 2011;43(5):543-551. 9. Carter RA, Engiles JB, Megee SO, et al. Decreased expression of p63, a regulator of epidermal stem cells, in the chronic laminitic equine hoof. Equine Vet J. 2011;43(5):543-551. 10. Carter RA, Shekk V, de Laat MA, et al. Novel keratins identified by quantitative proteomic analysis as the major cytoskeletal proteins of equine (Equus caballus) hoof lamellar tissue. J Anim Sci. 2010;88(12):3843-3855. 11. Clark RK, Galantino-Homer HL. Wheat Germ Agglutinin as a Counterstain for Immunofluorescence Studies of Equine Hoof Lamellae. Exp Dermatol. 2014. 12. Collins SN, Pollitt C, Wylie CE, et al. Laminitic pain: parallels with pain states in humans and other species. Veterinary Clinics of North America - Equine Practice. 2010;26(3):643-671. 13. Collins SN, van Eps AW, Pollitt CC, et al. The lamellar wedge. Vet Clin North Am Equine Pract. 2010;26(1):179-195. 14. de Laat MA, Clement CK, McGowan CM, et al. Toll-like receptor and pro-inflammatory cytokine expression during prolonged hyperinsulinaemia in horses: implications for laminitis. Vet Immunol Immunopathol. 2014;157(1-2):78-86. 15. de Laat MA, Clement CK, Sillence MN, et al. The impact of prolonged hyperinsulinaemia on glucose transport in equine skeletal muscle and digital lamellae. Equine Vet J. 2014. 16. de Laat MA, Sillence MN, McGowan CM, et al. Continuous intravenous infusion of glucose induces endogenous hyperinsulinaemia and lamellar histopathology in Standardbred horses. Vet J. 2012;191(3):317-322. 17. de Laat MA, van Eps AW, McGowan CM, et al. Equine laminitis: comparative histopathology 48 hours after experimental induction with insulin or alimentary oligofructose in standardbred horses. J Comp Pathol. 2011;145(4):399-409. 18. Eades SC. Overview of current laminitis research. Veterinary Clinics of North America: Equine Practice. 2010;26(1):51-63. 19. Eckert RE, Sharief Y, Jones SL. p38 mitogen-activated kinase (MAPK) is essential for equine neutrophil migration. Vet Immunol Immunopathol. 2009;129(3-4):181-191. 20. Engiles JB. Pathology of the distal phalanx in equine laminitis: more than just skin deep. Veterinary Clinics of North America - Equine Practice. 2010;26(1):155-165. 21. Epstein KL, Brainard BM, Giguere S, et al. Serial viscoelastic and traditional coagulation testing in horses with gastrointestinal disease. J Vet Emerg Crit Care (San Antonio). 2013;23(5):504-516. 22. Faleiros RR, Johnson PJ, Nuovo GJ, et al. Laminar leukocyte accumulation in horses with carbohydrate overload-induced laminitis. Journal of Veterinary Internal Medicine; 2011.25: 1, 107-115.30 ref. 2011. 23. Faleiros RR, Leise BS, Watts M, et al. Laminar chemokine mRNA concentrations in horses with carbohydrate overload-induced laminitis. Vet Immunol Immunopathol. 2011;144(1-2):45-51. 24. Faramarzi B. Morphological spectrum of primary epidermal laminae in the forehoof of Thoroughbred horses. Equine Vet J. 2011;43(6):732-736. 25. Galantino-Homer H, Carter R, Megee S, et al. The Laminitis Discovery Database. Journal of Equine Veterinary Science. 2010;30(2):101. 26. Garner HE, Coffman JR, Hahn AW, et al. Equine laminitis of alimentary origin: an experimental model. Am J Vet Res. 1975;36(4 Pt.1):441-444. 27. Grosenbaugh DA, Morgan SJ, Hood DM. The digital pathologies of chronic laminitis. Veterinary Clinics of North America - Equine Practice. 1999;15(2):419-436. 28. Harlow BE, Lawrence LM, Kagan IA, et al. Inhibition of fructan-fermenting equine faecal bacteria and Streptococcus bovis by hops (Humulus lupulus L.) beta-acid. J Appl Microbiol. 2014;117(2):329-339. 29. Heymering HW. 80 Causes, Predispositions, and Pathways of Laminitis. Vet Clin North Am Equine Pract. 2010;26(1):13-19. 30. Holbrook TC, Tipton T, McFarlane D. Neutrophil and cytokine dysregulation in hyperinsulinemic obese horses. Veterinary Immunology and Immunopathology; 2012.145: 1/2, 283-289.48 ref. 2012. 31. Jones E, Vinuela-Fernandez I, Eager RA, et al. Neuropathic changes in equine laminitis pain. Pain. 2007;132(3):321-331. 32. Kane A, Traub-Dargatz J, Losinger W, et al. cross-sectional survey of lameness and laminitis in US horses. 2000. 33. Kawasako K, Higashi T, Nakaji Y, et al. Histologic evaluation of the diversity of epidermal laminae in hooves of horses without clinical signs of laminitis. Am J Vet Res. 2009;70(2):186-193. 34. Knowles EJ, Menzies-Gow NJ, Mair TS. Plasma fructosamine concentrations in horses with pituitary pars intermedia dysfunction with and without laminitis. Equine Vet J. 2014;46(2):249-251. 35. Kullmann A, Holcombe SJ, Hurcombe SD, et al. Prophylactic digital cryotherapy is associated with decreased incidence of laminitis in horses diagnosed with colitis. Equine Vet J. 2013. 36. Kuwano A, Katayama Y, Yoshihara T, et al. A gross study of lamellar wedge development in equine laminitis. Journal of Equine Science. 2001;12(2):58. 37. Laat MAd, McGowan CM, Sillence MN, et al. Equine laminitis: induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet J. 2010;42(2):129-135. 38. Leise BS, Faleiros RR, Watts M, et al. Laminar inflammatory gene expression in the carbohydrate overload model of equine laminitis. Equine Vet J. 2011;43(1):54-61. 39. Leise BS, Watts M, Roy S, et al. Use of laser capture microdissection for the assessment of equine lamellar basal epithelial cell signalling in the early stages of laminitis. Equine Vet J. 2014. 40. Linardi R, Megee S, Senoo M, et al. Characterization of a stem cell-selective in vitro culture system for equine (Equus caballus) lamellar epidermal cells. Journal of Equine Veterinary Science. 2013;33(10):866-867. 41. Menzies-Gow NJ, Wray H, Bailey SR, et al. The effect of exercise on plasma concentrations of inflammatory markers in normal and previously laminitic ponies. Equine Vet J. 2014;46(3):317-321. 42. Moreau MM, Eades SC, Reinemeyer CR, et al. Illumina sequencing of the V4 hypervariable region 16S rRNA gene reveals extensive changes in bacterial communities in the cecum following carbohydrate oral infusion and development of early-stage acute laminitis in the horse. Vet Microbiol. 2014;168(24):436-441. 43. Morgan RA, McGowan TW, McGowan CM. Prevalence and risk factors for hyperinsulinaemia in ponies in Queensland, Australia. Aust Vet J. 2014;92(4):101-106. 44. Morgan SJ, Hood DM, Wagner IP, et al. Submural histopathologic changes attributable to peracute laminitis in horses. Am J Vet Res. 2003;64(7):829-834. 45. Nourian AR, Mills PC, Pollitt CC. Development of intraosseous infusion of the distal phalanx to access the foot lamellar circulation in the standing, conscious horse. Vet J. 2010;183(3):273-277. 46. Obel N. Studies on the histopathology of acute laminitis. 1948. 47. Onishi JC, Park JW, Prado J, et al. Intestinal bacterial overgrowth includes potential pathogens in the carbohydrate overload models of equine acute laminitis. Vet Microbiol. 2012;159(3-4):354-363. 48. Orsini JA, Parsons CS, Capewell L, et al. Prognostic indicators of poor outcome in horses with laminitis at a tertiary care hospital. Can Vet J. 2010;51(6):623-628. 49. Parsons CS, Orsini JA, Krafty R, et al. Risk factors for development of acute laminitis in horses during hospitalization: 73 cases (1997-2004). J Am Vet Med Assoc. 2007;230(6):885-889. 50. Pawlak E, Wang L, Johnson PJ, et al. Distribution and processing of a disintegrin and metalloproteinase with thrombospondin motifs-4, aggrecan, versican, and hyaluronan in equine digital laminae. Am J Vet Res. 2012;73(7):1035-1046. 51. Pawlak EA, Geor RJ, Watts MR, et al. Regulation of hypoxia-inducible factor-1alpha and related genes in equine digital lamellae and in cultured keratinocytes. Equine Vet J. 2014;46(2):203-209. 52. Pollitt CC, Visser MB. Carbohydrate alimentary overload laminitis. Veterinary Clinics of North America: Equine Practice. 2010;26(1):65-78. 53. Pollitt CC. The anatomy and physiology of the suspensory apparatus of the distal phalanx. Vet Clin North Am Equine Pract. 2010;26(1):29-49. 54. Pollitt CC. Anatomy and physiology of the inner hoof wall. (Special issue: Laminitis). Clinical Techniques in Equine Practice. 2004;3(1):3-21. 55. Rani S, Barbe MF, Barr AE, et al. Periostin-like-factor and Periostin in an animal model of workrelated musculoskeletal disorder. Bone. 2009;44(3):502-512. 56. Steelman SM, Johnson P, Jackson A, et al. Serum metabolomics identifies citrulline as a predictor of adverse outcomes in an equine model of gut-derived sepsis. Physiol Genomics. 2014;46(10):339-347. 57. van Eps AW, Pollitt CC. Equine laminitis model: lamellar histopathology seven days after induction with oligofructose. Equine vet J. 2009;41(1):00-00. 58. van Eps AW, Pollitt CC, Underwood C, et al. Continuous digital hypothermia initiated after the onset of lameness prevents lamellar failure in the oligofructose laminitis model. Equine Vet J. 2013. 59. Virgin JE, Goodrich LR, Baxter GM, et al. Incidence of support limb laminitis in horses treated with half limb, full limb or transfixation pin casts: a retrospective study of 113 horses (2000-2009). Equine Vet J Suppl. 2011;(40):7-11. doi(40):7-11. 60. Wang L, Pawlak E, Johnson PJ, et al. Effects of cleavage by a disintegrin and metalloproteinase with thrombospondin motifs-4 on gene expression and protein content of versican and aggrecan in the digital laminae of horses with starch gruel–induced laminitis. Am J Vet Res. 2012;73(7):1047-1056. 61. Wang L, Pawlak EA, Johnson PJ, et al. Expression and activity of collagenases in the digital laminae of horses with carbohydrate overload-induced acute laminitis. J Vet Intern Med. 2014;28(1):215-222. 62. Wang L, Pawlak EA, Johnson PJ, et al. Impact of laminitis on the canonical Wnt signaling pathway in basal epithelial cells of the equine digital laminae. PLoS One. 2013;8(2):e56025. 63. Wylie CE, Collins SN, Verheyen KL, et al. A cohort study of equine laminitis in Great Britain 20092011: estimation of disease frequency and description of clinical signs in 577 cases. Equine Vet J. 2013;45(6):681-687. 64. Wylie CE, Collins SN, Verheyen KL, et al. Frequency of equine laminitis: a systematic review with quality appraisal of published evidence. Vet J. 2011;189(3):248-256. 65. Zamboulis DE, Senior M, Clegg PD, et al. Expression of purinergic P2X receptor subtypes 1, 2, 3 and 7 in equine laminitis. Vet J. 2013;198(2):472-478. Figure 1. Normal foot- parasagittal section. DDFT, Deep Digital Flexor Tendon with insertion (asterisk); NAV, navicular bone; DC, digital cushion; MP, middle phalanx; DP, distal phalanx; white arrow and line, stratum medium; black arrow and line, stratum internum (lamellae with corium dermis). Figure 2. Chronically laminitic foot- parasagittal section. Severe rotation and sinking of the distal phalanx with subsolar abscess (arrow head); MP, middle phalanx; DP, distal phalanx; black arrow, lamellar wedge delineated by dorsal cortex of the DP (black line). Figure 3. Normal lamellae, H&E (2X, upper panel; 20X, lower panel). Interdigitating Primary Epidermal & Dermal Lamellae (PEL, PDL) merge abaxially with the stratum medium (SM). Secondary Epidermal & Dermal lamellae (SEL, SDL) extend from the keratinized axis (KA). Figure 4. Acute laminitis. H&E (2X, upper panel; 10X, lower panel). Necrosis with complete epidermal separation from the Primary Dermal Lamellae (PDL). Surrounding the keratinized axes (KA) are necrotic secondary epidermal lamellae (SEL) with early regeneration (lower right). Figure 5. Chronic laminitis. Complete abaxial epidermal separation (black arrow), dysplastic lamellar regeneration (white arrow) with hyperkeratosis (HK), acanthosis and epidermal islands (inset- A, asterisk).

![founder [foun-der] * verb](http://s3.studylib.net/store/data/006663793_1-d5e428b162d474d6f8f7823b748330b0-300x300.png)