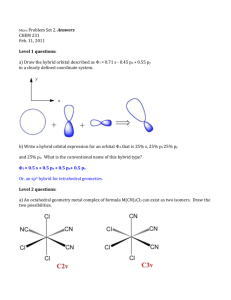
Quiz 2 Chemistry and Physics of Hybrids 1) Name three naturally
advertisement

Quiz 2 Chemistry and Physics of Hybrids 1) Name three naturally occurring hybrid organic-inorganic materials? a) nacre b) bone c) dentin d) enamel e) chitin exoskeletons of lobsters f) spider fangs 2) What are the inorganic and organic components in nacre (Mother of pearl)? Argonite and protein 3) What does hierarchical mean (in regards to materials)? Give an example of a hierarchical material. Different structures at different length scales (sizes). An example would be Nacre or bone or chitin exoskeletons. 4) Briefly describe how you could physically mix silica particles with an organic polymer like polystyrene? Melt the polystyrene and physically mix in the dry silica particles as a powder or Dissolve the polystyrene in solvent and mix in the particles also dispersed in solvent. Cast mixture and evaporate solvent. 5) What is the difference between intercalation and exfoliation when making claypolymer hybrids? Intercalation is the polymers replacing surfactant in the clay but leaving the stacked structure intact. Exfoliation is when the polymers unstack the 2-D sheets and disrupt the structure. 6) Give three examples of inorganic components physically mixed into hybrids: a) Silica particles b) Titanium dioxide c) fullerenes d) carbon nanotubes e) POSS 7) What is a nanocomposite hybrid organic inorganic material? Give an example of a nanocomposite. A hybrid with one or more of its phases is nanometer scale in size. An example would be nacre or POSS in polypropylene 8) List three examples of hybrid monomers that can be polymerized to give hybrid organic-inorganic materials? Organotrialkoxysilanes, dichlorosilanes, hexaachlorotriphosphazenes 8) What are three ways to make inorganic particles? Sol-gel polymerization, flame (aerosol) synthesis, emulsion polymerization, precipitation, electrochemistry, electroless reductions or oxidations 9) Describe the two main reactions that occur when metal alkoxide monomers, like organotrialkoxysilanes, polymerize? Hydrolysis: replacing alkoxide groups with OH groups Condensation: reaction of OH groups to form Si-O-Si groups 10) Why do gels form from hybrid monomers? Gels form because the hybrid monomer polymerizes into macromolecules with high enough molecular weight that they phase separate from solution as solid particles. This is called a sol. The solid particles gel when they are concentrated enough to percolate through the liquid.