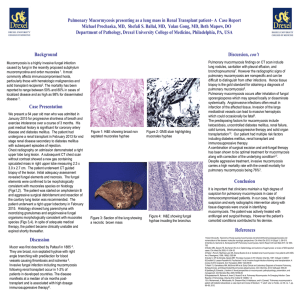
Early recognition and successful treatment of pulmonary
advertisement

Early recognition and successful treatment of pulmonary mucormycosis with a cavitary lesion in a renal transplant recipient Corresponding author: Tae-Won Lee. Author: Seon-Hye Kim, Chun-Gyu Ihm, Tae-Won Lee, Sang-Ho Lee, Kyung-Hwan Jeong, Mi Suk Lee, Joo-Young Moon Division of Nephrology, Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, South Korea Division of Infectious Diseases, Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, South Korea Abstract. Mucormycosis, or zygomycosis, is an opportunistic fungal infection that occurs characteristically in immunocompromised individuals, including those with diabetes mellitus, solid organ or hematopoietic stem cell transplantation, neutropenia, cancer and AIDS. The most common foci of this infection include the sinuses (39%), lungs (24%), and cutaneous tissues (19%). The infection becomes life-threatening when it invades the blood vessel endothelium and disseminates throughout the body. Dissemination may develop in 23% of cases. Herein we report a case of pulmonary mucormycosis with a cavitary lesion in a kidney transplant recipient with type 2 diabetes. This infection was discovered before it progressed and was treated successfully with a wedge resection and liposomal amphotericin B. Function in the newly received kidney was preserved. Therefore early recognition and appropriate therapy of this infection, before dissemination, can result in a favorable outcome. Keywords. Pulmonary mucormycosis, opportunistic fungal infection, renal transplant recipient, amphotericin B, amphotericin B lipid complex, surgical resection, early diagnosis and prompt treatment Introduction Mucormycosis is an opportunistic fungal infection, acquired through inhalation of spores, and typically manifests in the sinuses, lungs and gastrointestinal tract. The site of infection at the time of initial diagnosis may vary with characteristics of the host population. Predisposing factors for mucormycosis include immunocompromised status associated with diabetes, neutropenia, AIDS, malignancy and solid organ or hematopoietic stem cell transplantation. Among patients who undergo solid organ transplantation, 3%-4% develop pulmonary mucormycosis, by six months after transplantation [1]. One-half of the mycoses remain restricted to the lung, as a nodule or consolidation, or rarely as a cavitary lesion. However, the infection may progress to an invasive form that disseminates, to other organs causing high morbidity and mortality. Early diagnosis and prompt aggressive therapy may prevent progression and improve outcome. Pulmonary mucormycosis after kidney transplantation has been previously reported. The infection in our patient was unusual, however, because it was associated with a cavitary lesion, it began less than one month after kidney transplantation, and it was discovered and treated at an early stage. Thus we report here a case of pulmonary mucormycosis in a kidney transplant recipient with type 2 diabetes mellitus who was treated successfully with a wedge resection and liposomal amphotericin B. Case report A 48-year-old man with end stage renal disease secondary to diabetic nephropathy and on hemodialysis since 2006 underwent a living kidney transplantation on 7 December 2010. He was afterwards observed in our hospital as an outpatient on an immunosuppressive regimen that included tacrolimus (2 mg twice daily), prednisolone (15 mg daily, the dosage having been reduced from 20 mg), and mycophenolic acid (720 mg twice a day). Twenty days later, he was admitted to the hospital because of uncontrolled diabetes with no specific symptoms. Physical examination and initial laboratory data were not informative. Incidentally an initial chest X-ray showed a cavitary lesion (4.6 cm in diameter) on the right upper lobe (Fig. 1). Computed tomography (CT) of the chest (Fig. 2) showed a cavitary lesion 4.6 cm in diameter on the right upper lobe. The results of bronchoscopy suggested fungal pneumonia, tuberculosis or norcardiosis. Broncho-alveolar lavage (BAL) was performed with specific stains (e.g., Acid fast bacilli, Gomori methenamine silver stain), but neither culture nor cytology revealed any specific organism. Sputum culture, blood culture and serologic markers (including Aspergillus antigen) gave no clear diagnostic information. Computed tomography (CT) guided percutaneous needle aspiration and biopsy (PCNA) was conducted. The pathologic finding was acute inflammation, not helpful to diagnose. So we decided on surgical resection to determine the cause and to treat the lesion. Using video-assisted thoracic surgery (VATS), we performed a wedge resection, and pathologic examination revealed typical hyphae within the lung tissue (Fig. 3-A-C). This led to a diagnosis of pulmonary mucormycosis. After four days of intravenous amphotericin B (0.5 mg/kg daily), the patient’s serum creatinine level was increased from 0.8 mg/dL to 1.4 mg/dL. The antifungal agent was changed to liposomal amphotericin B (3 mg/kg, then increased to 4 mg/kg after seven days) and his serum creatinine level subsequently normalized. After the surgery, the patient had a running nose, which raised concern for development of sinus mucormycosis. Examination of para-nasal sinuses using paranasal CT showed mild mucosal thickening without fluid collection or soft tissue mass (Fig.4-A-C). On follow-up high resolution CT (HRCT) of lung (Fig.5) before 1 month in the postoperative period, small nodules on the lower lobe of the right lung were newly recognized as a fungal infection or pneumonia. With consistent use of liposomal amphotericin B, the small nodules disappeared. The patient was discharged from the hospital after 39 days of intravenous amphotericin B and a wedge resection. The immune-suppression regimen was modified to include prednisolone (reduced to 5 mg daily) and cyclosporine A (100 mg twice daily), and mycophenolic acid (reduced to 360 mg twice daily) and tacrolimus were discontinued. The patient’s renal function remained stable with a serum creatinine less than 1.2mg/dL after discharge from the hospital. Discussion Morbidity and mortality in the first year after renal transplantation occur most commonly through infection. The post-transplantation timetable is divided into three parts: the first month; one to six months; and more than six months thereafter. Infectious agents can often be identified by according to the time interval from renal transplantation to presentation. Bacterial and Candida infections predominate in the first month; these are related directly to the surgery. In the first six months after renal graft, high-dose immune-suppressive therapy makes the patient vulnerable to viral and opportunistic infections. Patients in the late post-transplantation period, develop mostly communityacquired infections, such as influenza and pneumococcal pneumonia, which are associated with the low maintenance dosage of immunosuppressive therapy. In contrast, the group of post-transplantation patients, maintained at a high level of immunosuppression, may develop opportunistic infections [4]. Pulmonary mucormycosis, an opportunistic fungal infection, usually occurs more than six months after transplantation. In association with diabetes mellitus, solid organ or hematopoietic stem cell transplantation, neutropenia or malignancy. Patients with multiple risk factors are accordingly more susceptible. Pulmonary mucormycosis usually presents as an infiltration or consolidation on radiological imaging. The presence of a cavitary lesion is relatively uncommon. The successful treatment of mucormycosis requires immediate use of antifungal agents and surgical resection. A recent study reported the benefit of early diagnosis and initiation of antifungal therapy. If treatment is initiated within the first five days after diagnosis of mucormycosis, the survival rate may be increased from 49% to 83%; this underscores the urgency for an early diagnosis [2, 7]. Amphotericin B, in conventional and liposomal formulations, remains the first-line agent for this disease, and the lipid formulation should be used in patients at risk for nephrotoxicity and can be administered at higher doses for a longer period [5]. Surgical removal of necrotic tissues may be essential for full recovery from mucormycosis. Recent studies show that patients who do not undergo surgical resection suffer higher mortality than those who do [8-11]. During the use of liposomal amphotericin B, we found newly developed lung nodules, concerned about progression of the infection, and considered use of salvage therapy. Posaconazole is a reasonable salvage option for patients with mucormycosis who refractory to polyenes. It appears quite safe and can be used much longer than other options [12-13]. But in our case, we didn’t use posaconazole for salvage therapy. The reasons are the concern about antagonistic interactions amphotericin B with posaconazole and the practical difficulty of getting posaconazole in our country. Pulmonary mucormycosis in our patient was discovered accidentally during evaluation for uncontrolled diabetes after renal graft. This suggests that a renal allograft recipient with a blood glucose level resistant to control should be closely observed for occult infection. The present case was distinguished by its detection, at less than one month after a kidney transplant and by the presence of a cavitary lesion. These features allowed us to treat the patient at the optimal stage for recovery, using antifungal drugs and surgery. Based on these observations, we strongly recommend that a kidney transplant recipient with diabetes be closely observed for opportunistic fungal infection to allow for prompt and aggressive treatment as needed. Figure 1. Initial chest X-ray showed a cavitary lesion (arrow) on the upper lobe of the right lung. Figure 2. Computed tomography of the chest revealed a right upper lobe cavitary lesion (arrow) that measured approximately 4.6 x 3.2 cm. Figure 3. Broad non-septate fungal hyphae have irregular branches. (A: hematoxylin and eosin stain, X 100). The fungal hyphae are long and irregularly branched. (B: PAS stain, X100). Non-septate hyphae, branched at right angles. (C: Gomori methenamine silver stain, X 400). Figure 4. Mild mucosal thickening of the paranasal sinuses was apparent, without fluid collection or soft tissue mass, on paranasal sinus computed tomography. Figure 5. HRCT (high resolution computed tomography) showed small nodules (arrows) on the lower lobe of the right lung. References [1] Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: A review of 929 reported Cases. Clin Infect Dis. 2005; 41:634-653. [2] Spellberg B, Ibrahim A, Recent advances in the treatment of mucormycosis. Curr Infect Dis Rep. 2010; 12:423–429. [3] Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives onmucormycosis: pathophysiology, presentation, and management. Clin Micro biol Rev. 2005; 18:556–569. [4] Sia IG, Paya CV. Infectious complications following renal transplantation. Surg Clin North Am. 1998; 78(1):95-112 [5] Almyroudis NG, Sutton DA, Linden P, Rinaldi MG, Fung J,Kusne S. Zygomycosis in solid organ transplant recipients in a tertiary transplant centre and review of the literature. Am J Transplant. 2006; 6: 2365–2374. [6] Tedder M, Spratt JA, AnstadtMP, Hegde SS, Tedder SD, LoweJE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994; 57:1044–1050. [7] Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008; 47:503–509. [8] Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis. 2000; 30:851–856. [9] Pavie J, Lafaurie M, Lacroix C, et al. Successful treatment of pulmonary mucormycosis in an allogenic bone-marrow transplant recipient with combined medical and surgical therapy. Scand J Infect Dis. 2004; 36:767–769. [10] Reid VJ, Solnik DL, Daskalakis T, Sheka KP. Management of bronchovascular mucormycosis in a diabetic: a surgical success. Ann Thorac Surg. 2004; 78:1449–1451. [11] Asai K, Suzuki K, Takahashi T, et al. Pulmonary resection with chest wall removal and reconstruction for invasive pulmonary mucormycosis during anti-leukemia chemotherapy. Jpn J Thorac Cardiovasc Surg. 2003; 51:163–166. [12] Rickerts V, Böhme A, Just-Nübling G. Risk factor for invasive zygomycosis in patients with hematologic malignancies [in German]. Mycoses 2002, 45(Suppl 1):27–30. [13] Dannaoui E, Meis JF, Loebenberg D, Verweij PE. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob Agents Chemother 2003, 47:3647–3650. [14] Walsh TJ, Kontoyiannis DP. What is the role of combination therapy in management of zygomycosis? Clin Infect Dis 2008; 47:372–4.