Renal Cortical Pyruvate Depletion during AKI
advertisement

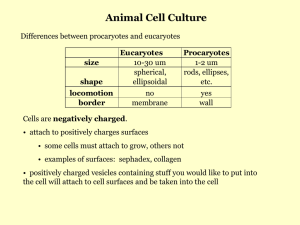
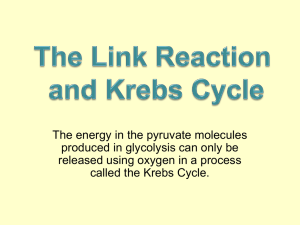
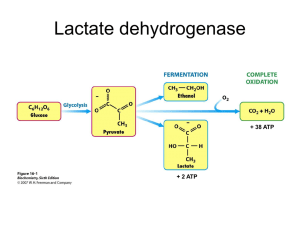
A - Organic ARF Renal Cortical Pyruvate Depletion during AKI Richard A. Zager, Ali C.M. Johnson and Kirsten Becker JASN May 2014 vol. 25 no. 5 : 998-1012 + Author Affiliations Fred Hutchinson Cancer Research Center, University of Washington, Seattle, Washington Correspondence: Dr. Richard A. Zager, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue N, Room D2-190, Seattle, WA 98109. Email: dzager@fhcrc.org ABSTRACT Pyruvate is a key intermediary in energy metabolism and can exert antioxidant and antiinflammatory effects. However, the fate of pyruvate during AKI remains unknown. Here, we assessed renal cortical pyruvate and its major determinants (glycolysis, gluconeogenesis, pyruvate dehydrogenase [PDH], and H2O2 levels) in mice subjected to unilateral ischemia (15– 60 minutes; 0–18 hours of vascular reflow) or glycerol-induced ARF. The fate of postischemic lactate, which can be converted back to pyruvate by lactate dehydrogenase, was also addressed. Ischemia and glycerol each induced persistent pyruvate depletion. During ischemia, decreasing pyruvate levels correlated with increasing lactate levels. During early reperfusion, pyruvate levels remained depressed, but lactate levels fell below control levels, likely as a result of rapid renal lactate efflux. During late reperfusion and glycerol-induced AKI, pyruvate depletion corresponded with increased gluconeogenesis (pyruvate consumption). This finding was underscored by observations that pyruvate injection increased renal cortical glucose content in AKI but not normal kidneys. AKI decreased PDH levels, potentially limiting pyruvate to acetyl CoA conversion. Notably, pyruvate therapy mitigated the severity of AKI. This renoprotection corresponded with increases in cytoprotective heme oxygenase 1 and IL-10 mRNAs, selective reductions in proinflammatory mRNAs (e.g., MCP-1 and TNF-α), and improved tissue ATP levels. Paradoxically, pyruvate increased cortical H2O2 levels. We conclude that AKI induces a profound and persistent depletion of renal cortical pyruvate, which may induce additional injury. COMMENTS A key point in the pathophysiology of Acute Kidney Insufficiency, demonstrated using ischemic protocols, glycerol-induced protocol and using Pyruvate therapy in mice. Increasing evidence indicates the diverse role that pyruvate can play as a potential modulator of tissue injury. Pyruvate is classically viewed as a key determinant of cellular energy metabolism. Under aerobic conditions, pyruvate, generated from glucose by glycolysis, undergoes decarboxylation by the enzyme complex pyruvate dehydrogenase (PDH), forming acetyl CoA. Alternatively, pyruvate can be carboxylated by pyruvate carboxylase, yielding oxaloacetate, with subsequent acetyl CoA/oxaloacetate metabolism through the Krebs cycle and increased aerobic ATP production results. Conversely, under anaerobic conditions, pyruvate is metabolized by lactate dehydrogenase (LDH) to lactate, and in the process, NAD is generated, which is required for additional glycolytic ATP production. In addition to its central role in supporting both aerobic and anaerobic energy production, pyruvate may exert a variety of cytoprotective effects. Pyruvate is a potent H2O2 scavenger, which was assessed in an in vitro system. Furthermore, pyruvate administration decreased renal injury after intrarenal H2O2 injection and during the course of the glycerol model of rhabdomyolysis-induced ARF. In addition to its antioxidant properties, pyruvate may also exert anti-inflammatory and cytoprotective effects against experimental ischemic-reperfusion injury and shock. The goals of this study were threefold: first, document the influence of evolving ischemic and toxic AKI on pyruvate levels within renal cortex; second, ascertain the metabolic pathways that ultimately alter pyruvate expression; third, further elucidate the potential effects of pyruvate on the course and selected potential mediators of experimental models of ARF. Finally, given that pyruvate administration can attenuate AKI, it seems reasonable to postulate that pyruvate depletion as defined by this study is not merely a benign consequence of ischemic or toxic renal injury; rather, it likely plays a significant pathophysiologic role in their development.