CURRICULUM SUMMARY –Autumn Term (September
advertisement

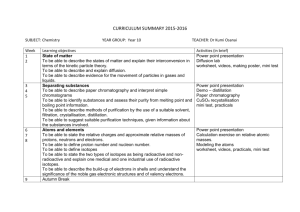
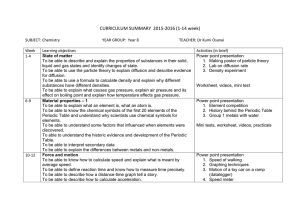
CURRICULUM SUMMARY –Autumn Term (September-December) 2014 SUBJECT: Chemistry Week 1 2 3 4 5 6 7 8 9 YEAR GROUP: Year 10 TEACHER: Dr Kumi Osanai Learning objectives State of matter To be able to describe the states of matter and explain their interconversion in terms of the kinetic particle theory. To be able to describe and explain diffusion. To be able to describe evidence for the movement of particles in gases and liquids. Separating substances To be able to describe paper chromatography and interpret simple chromatograms To be able to identify substances and assess their purity from melting point and boiling point information. To be able to describe methods of purification by the use of a suitable solvent, filtration, crystallisation, distillation. To be able to suggest suitable purification techniques, given information about the substances involved. Activities (in brief) Power point presentation Diffusion lab worksheet, videos, making poster, mini test Atoms and elements To be able to state the relative charges and approximate relative masses of protons, neutrons and electrons. To be able to define proton number and nucleon number. To be able to define isotopes To be able to state the two types of isotopes as being radioactive and nonradioactive and explain one medical and one industrial use of radioactive isotopes. To be able to describe the build-up of electrons in shells and understand the significance of the noble gas electronic structures and of valency electrons. Power point presentation Calculation exercise on relative atomic masses. Modeling the atoms worksheet, videos, practicals, mini test Power point presentation Demo – distillation Paper chromatography CuSO4 recystallisation mini test, practicals 10 11 12 13 14 15 16 17 Atoms combining To be able to describe the differences between elements, mixtures and compounds, and between metals and non-metals. To be able to describe an alloy, such as brass, as a mixture of a metal with other elements. To be able to describe the formation of ions by electron loss or gain. To be able to describe the formation of ionic bonds between elements from Group I and VII. To be able to describe the formation of single covalent bonds in H2, Cl2, H2O, CH4, and HCl as sharing of pairs of electrons. To be able to describe the giant covalent structures of graphite and diamond. Reacting masses, and chemical equations Power point presentation Modeling the ionic compounds worksheet, videos, practicals, mini test Power point presentation Calculation in mole concept worksheet, videos, practicals, mini test