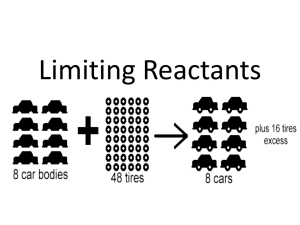
SCH 3U Limiting Reagents and Percent Yield Worksheet Given the
advertisement

SCH 3U 1. Limiting Reagents and Percent Yield Worksheet Given the following balanced chemical equation, answer the questions below. 2 AlCl3(aq) + 3 Na2S(aq) Al2S3(s) + 6 NaCl(aq) a) Name the two products of the reaction above. b) Assume you have mixed 45.0 g of aluminum chloride in water to make an aqueous solution and have made another aqueous solution containing 55.0 g of sodium sulfide. The solutions are mixed together. Use this information and your balanced chemical equation from above to: i) do the math required to identify the limiting reagent and the reagent that is in excess. ii) calculate the theoretical yield of the precipitate in the reaction. c) When the reaction described in part c) is completed in the lab, 20.0 g of the precipitate is actually collected. Use the theoretical yield from part c) to help you calculate the % yield for this reaction under these circumstances. d) How efficient would you say the reaction described above is under these conditions? e) Use pages 314 – 316 in the McGraw-Hill Ryerson text to list some reasons why the percent yield and the theoretical yield are not the same. f) Assume another trial of the reaction above is performed. This time the percent yield for the reaction is 110%. Use pages 314 – 316 in the McGraw-Hill Ryerson text to list some reasons why the percent yield and the theoretical yield are not the same 2. Given the following balanced chemical equation, answer the questions below. LiOH(aq) + AgNO3(aq) LiNO3(aq) + AgOH(s) a) b) c) d) 3. Assume that 100.0 g of lithium hydroxide in an aqueous solution and 200.0 g of silver nitrate in an aqueous solution are mixed together. Name the two products of the reaction above. Determine which reactant is the limiting reagent and which reactant is in excess. Show your work. Calculate the theoretical yield of the silver containing product. If you actually obtain 5.20 g less product than predicted by the theoretical yield, what is the % yield for this reaction? 150.0 g of ammonium sulfate is added to 250.0 g of vanadium(V) hydroxide. Both reactants are in aqueous solution. The products of this double displacement reaction are a precipitate containing vanadium(V) and an ionic compound in aqueous solution. a) Write a balanced chemical equation for the reaction. Use the information above to write in the physical state of each reactant and product. [Answer: 5 (NH4)2SO4(aq) + 2 V(OH)5(aq) V2(SO4)5(s) + 10 NH4OH(aq)] b) Name the two products of the reaction above. c) Determine which reactant is the limiting reagent and which reactant is in excess. Show your work. d) What is the theoretical yield of the vanadium(V) containing product? e) If you obtain 20.0 g of the vanadium(V) containing product after mixing the two reactants in the lab, what is the % yield for this reaction? The following two problems may not look like limiting reagent problems, but they are. We know this because information is given about more than one reactant and a question is asked about how much product will be formed. The only way to determine how much product will be formed is to first determine which of the reactants will be completely used up (the limiting reagent) because it is the information from this reactant that must be used to predict the theoretical yield of product. 4. What mass of CO2 will be produced under ideal conditions when 8.50 g of CH4 are placed in a reaction vessel with 15.9 g of O2? CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g) ? 5. (10.9 g) Calculate the theoretical yield of Fe2O3 produced when 20.9 g of FeS are placed in a reaction vessel with 14.1 g of O2 . 4 FeS(s) + 7 O2 (g) 2 Fe2O3 (s) + 4 SO2 (g) (18.9 g)