2 property of fluids: density
advertisement

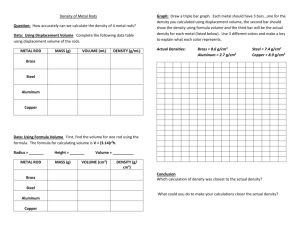
#2 PROPERTY OF FLUIDS: DENSITY GO #3 • observe the mass and volume of a liquid, and calculate its density using the formula d = m/v • compare densities of materials; and explain differences in the density of solids, liquids and gases, using the particle model of matter Each pure substance has its own density that will never change. It is a property that helps identify a substance. 1. DENSITY is _____________________________________________________________. 2. The formula for DENSITY is ________________________________________________ Or 3. Units: Density = D = M V -mass is measured in __________________ (g) -volume of solids is _____________________(cm3) -volume of fluids is in __________________(mL) therefore: -density of solids units are ___________/___________ -density of liquids units are ___________/_____________ 4. If the volume of a substance is 15.1 cm3 and its mass is 40.77g, what is its density? 5. The substance above is aluminum. The density means that for every cubic centimeter (cm3) it would have a mass of _____g 6. Calculate the density of a rock if its volume was 25.0 cm3 and it had a mass of 70.0 g. 7. A block of metal has a mass of 90 g and a volume of 10.1 cm3. What is it’s density? 8. What is the density of a piece of metal with a mass of 59.8g and a volume of 2.08cm3? 9. What is the density of a 39.6g substance that is 9cm3? CALCULATING DENSITY OF OBJECTS: FIRST YOU NEED TO FIND IT’S MASS AND VOLUME 10. Describe how you would find the mass of a solid cube. 11. Describe how you would find the mass of a fluid. (page 44) 12. Describe how you would find the volume of a solid cube or rectangular prism. (formula- math textbook page 252) 13. Describe how you would find the volume of a fluid. (page 424) CALCULATE THE DENSITY OF EACH OF THE FOLLOWING ITEMS: FIRST YOU WILL HAVE TO CALCULATE THE MASS AND VOLUME OF THE OBJECT. 14. Calculate out the density of the three different density blocks supplied. Show all your work. Do one wood. Once done calculating compare your density answers to the answer sheet to decide what each block is made of? BLOCK # & MASS VOLUME DENSITY MADE DESCRIPTION (g) CALCULATIONS CALCULATIONS OF ? (cube-cm3) (formula-show work) Look at sheet 15. Calculate out the density of water. Give details on how you calculated mass and volume of these items. WATER MASS VOLUME DENSITY CALCULATIONS AMOUNT (grams) (formula-show work) 40 ml 80 ml 100 ml 16. Graph the volume-mass relationship of water you worked on. Then plot the point for each of your density blocks using an asterisk for each. Connect each density point to the origin (0,0) (label each block in a legend). See page 43. VOLUME vs. MASS CALCULATING DENSITY FROM A GRAPH: 17. Example #1: Looking at the graph on page 43 you can see that 90cm3 of wood (pine) has a mass of 45g. Step 1: write the formula: D=M/V Step 2: choose a volume (ie: 40cm3) from the graph Step 3: find the mass for the chosen volume *a volume of 40cm3 has the mass of 20g* Step 4: substitute data into the formula and calculate the density so the density is (D=M/V) D=20g/40cm3 =0.5g/cm3 Step 5: take another volume and mass to check your calculations (You would get the same density calculation) 18. Calculate the density of isopropyl alcohol and vegetable oil by taking two different volumes with the mass off the graph on page 43. SHOW YOUR WORK!! ISOPROPYL ALCOHOL VEGETABLE OIL Use the chart to answer the following questions. SHOW WORK APPROXIMATE DENSITIES OF COMMON MATERIALS FLUID DENSITY SOLIDS DENSITY (g/mL) (g/cm3) Helium 0.0002 Wood(western red 0.37 cedar) Nitrogen 0.00125 Wood (birch) 0.66 Air 0.0013 Sugar 1.59 Oxygen 0.0014 Salt 2.16 Carbon dioxide 0.002 Aluminum 2.70 Isopropanol 0.79 Iron 7.87 Vegetable oil 0.9 Nickel 8.90 (varies) Water 1.00 Copper 8.92 Glycerol 1.26 Lead 11.34 Mercury 13.55 Gold 19.32 19. For a substance to be very dense it would have a _HIGH OR LOW_density. 20. What is the most dense substance in the table? 21. What is the least dense substance in the table? 22. Name two gases that are less dense than air. 23. Name two gases that are denser than air. 24. Each substance has its own specific density. What substance has a density of 8.92g/cm3? THIS MEANS THAT FOR EACH 1cm3 of this substance it would have a mass of ______g. 25. Vegetable oil has a density of _______, so if you had 10 mL of vegetable oil how much would its mass be? 26. If you had 100 cm3 of gold, what would its mass be? Use the formula to answer the next questions. Show your work. 27. What is the density of a substance with a mass of 50.6 g and a volume of 18.74 cm3? 28. If the volume of a substance is 15.1 cm3 and its mass is 8.3 g, what is its density? 29. The mass is 142.9 g, the volume is 12.6 cm3. What is the density? 30. Knowing that each substance has it’s own density, from #27, #28 and #29 which one was wood? _____ aluminum?_____ lead? ______ (look at the chart.) 31. A block of metal has a mass of 90 g and a volume of 10.1 cm3. What metal might it be? 32. A block of an unknown metal is 5cm X 3cm X 2cm. The block has a mass of 235g. What metal do you think the block is made of? Use your work to support your answer. 33. Looking at the substances graphed on page 43, what can you say about the density of a substance and the slope of the line when graphed?