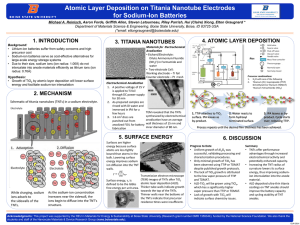
Venugopal Thayanithy_Abstract_DOM2015
advertisement

Long-distance intercellular transport of microRNAs via tunneling nanotubes: role in tumor-stroma interactions and malignant potential Venugopal Thayanithy1, Elizabeth Dickson1, Subbaya Subramanian3, Clifford J. Steer2, Emil Lou1 Division of Hematology Oncology and Transplantation1 Department of Medicine2, Department of Surgery3 University of Minnesota, Minneapolis, MN Tunneling nanotubes (TnTs) are long narrow actin-based cytoplasmic extensions which serve as unique structures facilitating direct long distance cell-to-cell transport of cargo. We hypothesized that microRNA can be transported between malignant and stromal cells via TnTs. miR-19a was labelled with Alexa-488 by in vitro transcription, and K7M2 (murine osteosarcoma) cells were reverse transfected prior to co-culture with K7M2 or DiO stained MC3T3 murine osteoblasts and imaged by time lapse microscopy. After 48 hr co-culture recipient cells were collected and analyzed by FACS. TnT-mediated cell-to-cell transport of miR-19a was observed in K7M2 cells themselves and between K7M2 and MC3T3 osteoblasts in TnTs spanning 40-200 nm in length, occurring over 25 minutes to few hours. Upon co-culture with transfected K7M2 cells for 48 hrs, relative levels of miR-19a were elevated up to 2.4-fold in MC3T3 osteoblast cells and a 0.4 µm membrane barrier reduced the double positive cells from 44.8% to 16.4%. Similar experiments in human ovarian cancer and ovarian epithelial cells also confirmed the transport of miR-199a between human ovarian cancer (SKOV3 and C200) and normal human ovarian epithelial(IOSE) cells. To our knowledge, we provide the first demonstration of intercellular transport of miRNAs between cancer and stromal cells via TnTs. We also provide proof that TnTs exist in human ovarian tumors and orthotopic osteosarcoma tumors. This discovery establishes a novel mechanism by which transport of miRNAs can mediate altered gene regulation in the complex and heterogeneous tumor microenvironment.