January 28, 2013
advertisement
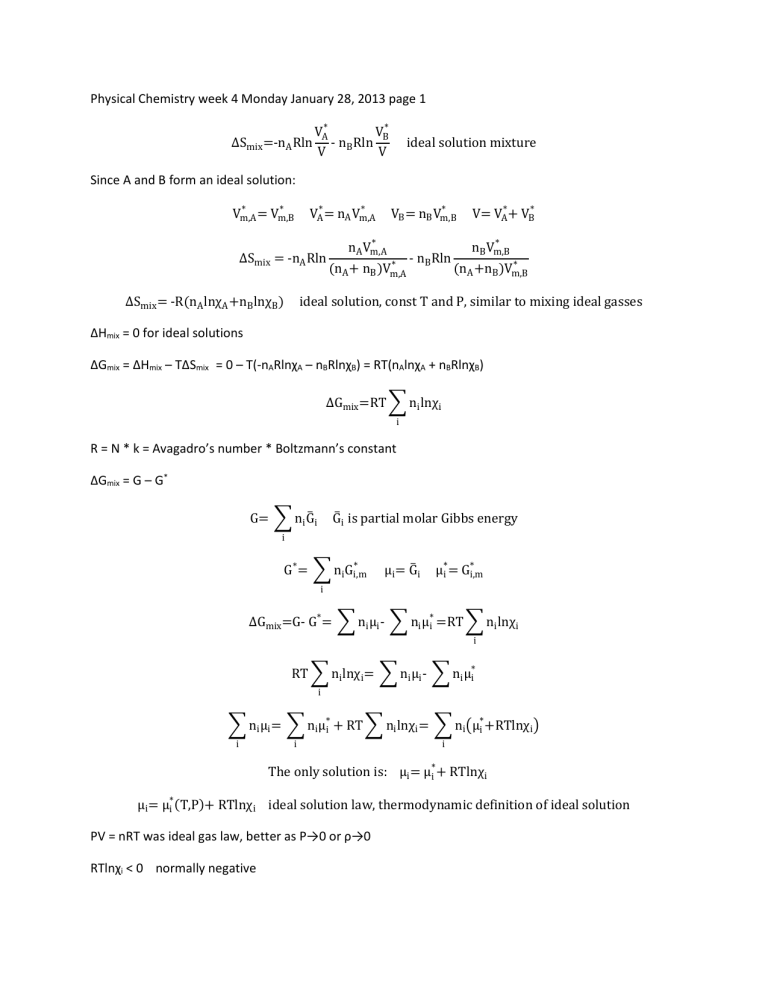
Physical Chemistry week 4 Monday January 28, 2013 page 1 ∆Smix =-nA Rln VA* VB* - nB Rln V V ideal solution mixture Since A and B form an ideal solution: * * Vm,A = Vm,B * VA* = nA Vm,A ∆Smix = -nA Rln ∆Smix = -R(nA lnχA +nB lnχB ) * VB = nB Vm,B * nA Vm,A * (nA + nB )Vm,A - nB Rln V= VA* + VB* * nB Vm,B * (nA +nB )Vm,B ideal solution, const T and P, similar to mixing ideal gasses ΔHmix = 0 for ideal solutions ΔGmix = ΔHmix – TΔSmix = 0 – T(-nARlnχA – nBRlnχB) = RT(nAlnχA + nBRlnχB) ∆Gmix =RT ∑ ni lnχi i R = N * k = Avagadro’s number * Boltzmann’s constant ΔGmix = G – G* G= ∑ ni G̅ i G̅ i is partial molar Gibbs energy i * G* = ∑ ni Gi,m μi = G̅ i * μ*i = Gi,m i ∆Gmix =G- G* = ∑ ni μi - ∑ ni μ*i =RT ∑ ni lnχi i RT ∑ ni lnχi = ∑ ni μi - ∑ ni μ*i i ∑ ni μi = ∑ ni μ*i + RT ∑ ni lnχi = ∑ ni (μ*i +RTlnχi ) i i i The only solution is: μi = μ*i + RTlnχi μi = μ*i (T,P)+ RTlnχi ideal solution law, thermodynamic definition of ideal solution PV = nRT was ideal gas law, better as P→0 or ρ→0 RTlnχi < 0 normally negative so μi < μ*i μ*i is constant As χi →0, µi →-∞ at constant T,P Thermodynamic properties of ideal solutions a. standard states For gas: pure ideal gas, P°=1 bar, T = Tinterest For liquid solutions: pure liquid(before mixing), T = Tsoln, P=Psoln For solid solutions: pure solid, T=Tsoln, P=Psoln μ°i is chemical potential of i at standard state 1 ∴ μ°i = μ*i (T,P) ° means standard state *means pure substance 2 μi = μ*i + RTlnχi Statements 1 and 2 are definition of ideal solution ∆Gmix =RT ∑ ni lnχi pressure independent i Since 0 < χi < 1 then lnχi <0 and ΔGmix < 0 so mixing is irreversible(spontaneous) dG = VdP – SdT dT = 0 dG = VdP ∂G V= ( ) ∂P T ∆Vmix = ( ∂∆Gmix ) ∂P T,ni Since ∆Gmix is independent of P: ∴ ∆Vmix =0 constant T and P ∂∆Gmix ∂ ∆Smix = - ( ) = - (RT ∑ ni lnχi ) ∂T P,ni ∂T i ∂∆Gmix ( ) =0 ∂P T,ni = -R ∑ ni lnχi ideal solution P,ni Since lnχi < 0, ΔSmix > 0 usually Exception: diethyl amine + water: ΔSmix<0, not really ideal since water is much smaller molecule ΔGmix = ΔHmix – TΔSmix so ΔHmix = ΔGmix + TΔSmix ∆Hmix =RT ∑ ni lnχi -RT ∑ ni ln χi =0 ΔHmix = 0 for ideal solutions When TΔS is at max, ΔG is at min On graph of mixing quantities versions mole fraction of substance B, the slope is steepest at the edges, which is why it’s hard to purify a substance out to 99.9999% purity. As an example, it’s not worth the trouble to extract gold that’s dissolved in the ocean. For ideal solutions: ΔHmix = 0 ΔVmix = 0 If a solution has ΔHmix = 0 and ΔVmix = 0 that’s not enough to conclude that it’s an ideal solution. Immiscible doesn’t count as a solution. For a “real” solution, ΔGmix <0 (always!)