October 22, 2012
advertisement
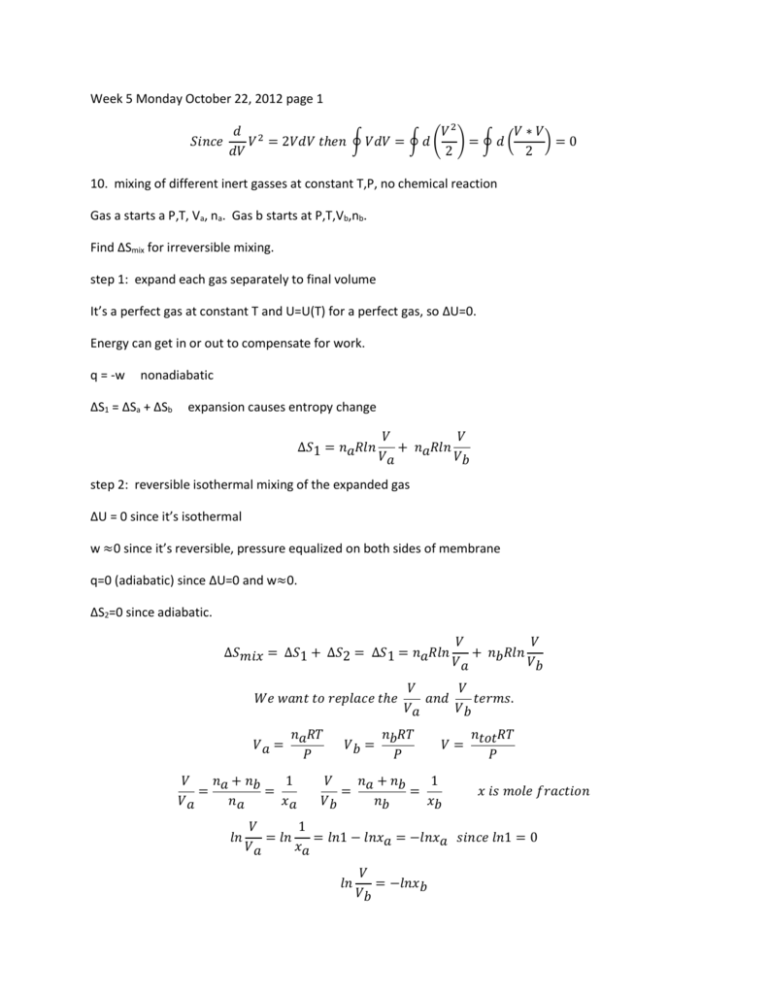
Week 5 Monday October 22, 2012 page 1 𝑑 2 𝑉2 𝑉∗𝑉 𝑆𝑖𝑛𝑐𝑒 𝑉 = 2𝑉𝑑𝑉 𝑡ℎ𝑒𝑛 ∮ 𝑉𝑑𝑉 = ∮ 𝑑 ( ) = ∮ 𝑑 ( )=0 𝑑𝑉 2 2 10. mixing of different inert gasses at constant T,P, no chemical reaction Gas a starts a P,T, Va, na. Gas b starts at P,T,Vb,nb. Find ∆Smix for irreversible mixing. step 1: expand each gas separately to final volume It’s a perfect gas at constant T and U=U(T) for a perfect gas, so ∆U=0. Energy can get in or out to compensate for work. q = -w nonadiabatic ∆S1 = ∆Sa + ∆Sb expansion causes entropy change ∆𝑆1 = 𝑛𝑎𝑅𝑙𝑛 𝑉 𝑉 + 𝑛𝑎𝑅𝑙𝑛 𝑉𝑎 𝑉𝑏 step 2: reversible isothermal mixing of the expanded gas ∆U = 0 since it’s isothermal w ≈0 since it’s reversible, pressure equalized on both sides of membrane q=0 (adiabatic) since ∆U=0 and w≈0. ∆S2=0 since adiabatic. 𝑉 𝑉 ∆𝑆𝑚𝑖𝑥 = ∆𝑆1 + ∆𝑆2 = ∆𝑆1 = 𝑛𝑎𝑅𝑙𝑛 + 𝑛𝑏𝑅𝑙𝑛 𝑉𝑎 𝑉𝑏 𝑊𝑒 𝑤𝑎𝑛𝑡 𝑡𝑜 𝑟𝑒𝑝𝑙𝑎𝑐𝑒 𝑡ℎ𝑒 𝑉𝑎 = 𝑛𝑎𝑅𝑇 𝑃 𝑉 𝑛 + 𝑛𝑏 1 = 𝑎 = 𝑉𝑎 𝑛𝑎 𝑥𝑎 𝑙𝑛 𝑉𝑏 = 𝑉 𝑉 𝑎𝑛𝑑 𝑡𝑒𝑟𝑚𝑠. 𝑉𝑎 𝑉𝑏 𝑛𝑏𝑅𝑇 𝑃 𝑉= 𝑉 𝑛 + 𝑛𝑏 1 = 𝑎 = 𝑉𝑏 𝑛𝑏 𝑥𝑏 𝑛𝑡𝑜𝑡𝑅𝑇 𝑃 𝑥 𝑖𝑠 𝑚𝑜𝑙𝑒 𝑓𝑟𝑎𝑐𝑡𝑖𝑜𝑛 𝑉 1 = 𝑙𝑛 = 𝑙𝑛1 − 𝑙𝑛𝑥𝑎 = −𝑙𝑛𝑥𝑎 𝑠𝑖𝑛𝑐𝑒 𝑙𝑛1 = 0 𝑉𝑎 𝑥𝑎 𝑙𝑛 𝑉 = −𝑙𝑛𝑥𝑏 𝑉𝑏 ∆𝑆𝑚𝑖𝑥 = −𝑛𝑎𝑅𝑙𝑛𝑥𝑎 − 𝑛𝑏𝑅𝑙𝑛𝑥𝑏 ∆𝑆𝑚𝑖𝑥 = −𝑅(𝑛𝑎𝑙𝑛𝑥𝑎 + 𝑛𝑏𝑙𝑛𝑥𝑏) 𝑅 = 8.314 𝐽 𝑚𝑜𝑙 𝐾 ∆Smix > 0 since lnxa < 1 and lnxb < 1 ∆𝑆𝑚𝑖𝑥 = −𝑅(𝑛𝑎𝑙𝑛𝑥𝑎 + 𝑛𝑏𝑙𝑛𝑥𝑏) 𝑝𝑒𝑟𝑓𝑒𝑐𝑡 𝑔𝑎𝑠, 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡 𝑇, 𝑃, 𝑎 ≠ 𝑏 If a = b then ∆Smix = 0. ∆Smix = -r(nalnxa + nblnxb) = -2R(nalnxa) but xa = 1 so lnxa = 0 ∆Smix = 0 Entropy: reversibility and irreversibility 1. reversible process ∆S = ∆Ssys ∆Ssys + ∆Ssurr = ∆Suniv 𝑑𝑆𝑢𝑛𝑖𝑣 = 𝑑𝑆𝑠𝑦𝑠 + 𝑑𝑆𝑠𝑢𝑟𝑟 = 𝑑𝑞𝑟𝑒𝑣 −𝑑𝑞𝑟𝑒𝑣 + =0 𝑇 𝑇 ∆Suniv = 0 for a reversible process Ssys + Ssurr = Suniv so Suniv remains constant for a reversible process reversible process is an idealization 2. irreversible process (adiabatic) finite number of steps Once we get to step 2, we can’t go back to step 1. Process from step 2 to 3 is reversible adiabatic compression (T rises). Process from step 3 to 4 is reversible isothermal compression. Process from step 4 to 1 is reversible adiabatic expansion. Thr is temperature of hot (heat) reservoir. S2 – S1 = ∆Ssys(irrev) = ? ∆S2→3 = 0 since adiabatic S3 - S2 = 0 S3 = S2 3→4 let heat in to maintain T but also to get entropy at point 4 to match point 1. 4 𝑆4 − 𝑆3 = ∫ 3 4 𝑑𝑞𝑟𝑒𝑣 1 𝑞 = ∫ 𝑑𝑞𝑟𝑒𝑣 = 3 → 4 𝑇 𝑇ℎ𝑟 3 𝑇ℎ𝑟 ∮ 𝑑𝑆𝑠𝑦𝑠 = (𝑆2 − 𝑆1) + (𝑆3 − 𝑆2) + (𝑆4 − 𝑆3) + (𝑆1 − 𝑆4) = 0 , (𝑆3 − 𝑆2) = 0 , (𝑆1 − 𝑆4) = 0 ∮ 𝑑𝑆𝑠𝑦𝑠 = (𝑆2 − 𝑆1) + (𝑆4 − 𝑆3) = 0 𝑞 𝑆2 − 𝑆1 + 3 → 4 𝑇ℎ𝑟 𝑑𝑒𝑝𝑒𝑛𝑑𝑠 𝑜𝑛 𝑤ℎ𝑒𝑡ℎ𝑒𝑟 𝑞 𝑖𝑠+, −, 𝑜𝑟 0 ∮ 𝑑𝑈 = 0 𝑠𝑜 ∮(𝑑𝑞 + 𝑑𝑤) = 0 𝑎𝑛𝑑 ∮ 𝑑𝑞 + ∮ 𝑑𝑤 = 0 ∮ 𝑑𝑞 𝑖𝑠 𝑞3 w = -q3→4 (system) similarly: -w = q3→4 →4 𝑠𝑜 𝑞3 →4 +𝑤 = 0 w is total work done on the system -w is work done by the surroundings Case 1: q3→4 is positive Then –w > 0 or w < 0 (work done by the system) This violates the second law 1→2→3→4→1 so q3→4 has to be ≤ 0. 𝑆2 − 𝑆1 = −𝑞3 4 → ≥0 𝑇ℎ𝑟 -q3→4 ≥ 0 Suppose –q3→4 = 0. Then q3→4 = 0 and w3→4 = 0. Then dV = 0, which would mean points 3 and 4 are the same point. So q3→ < 0 (only choice left)