Lab 9
advertisement

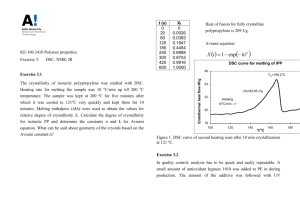
“Polypropylene Phase Transitions Studied by Differential Scanning Calorimetry” Ralph Eachus Submitted 3/17/2014 Chem 457 Section 2 TA: Chris Li Abstract: The structure of amorphous polypropylene dictates that it cannot become crystalline like isotactic polypropylene. Due to the difference in these two molecules, amorphous polypropylene undergoes a glass phase transition while isotactic polypropylene undergoes a first order transition of melting and freezing. A sample of each was tested using Differential Scanning Calorimetry and the thermograms produced were compared. The amorphous sample proved to be impure and contained 20.66% crystallinity and was therefore labeled incorrectly by the manufacturer. The heat of fusion of the isotactic sample was 86.97 J/g and proved to be consistent with the literature value. The glass phase transition temperature of the amorphous sample was -13.87 °C which differed from the literature value by 7°C due to the presence of isotactic polypropylene impurity. The change in entropy associated with the glass phase transition temperature of the amorphous sample was -28.16 J/g oC. Introduction: Differential Scanning Calorimetery (DSC) is a common method used to measure heat capacity, heat of transition as well as the temperature of phase changes.2 These calorimeters work by linearly heating a pan with the sample being tested and comparing it to a reference pan that is also being heated. Each pan is heated by a separate heater but their temperatures are kept equal at all times by thermocouples. The difference in energy required to maintain the sample at the same temperature as the reference is the measured heat flow.1 The computer attached to the calorimeter will plot the difference in energy output of the two heaters vs. the temperature.3 The extra energy output is the energy absorbed by the sample that is being tested. When the energy output is divided by the heating rate it yields the heat capacity (Cp) of the sample. When there is no chemical or physical change, the heat capacity is assumed to be independent of temperature but when there is a chemical or physical change, the excess heat added is the change in heat capacity.1 Cp = qp / ΔT (1) qp is the excess heat added to the sample being tested. A physical or chemical change such as melting is characterized by a peak in a thermogram.1 Peaks represent first order phase transitions where the sample completely changes phase; for example solid to liquid. The enthalpy (H) change associated with such a phase transition can be calculated using the starting and ending temperatures of the transition: 𝑇 𝛥𝐻 = ∫𝑇 2 𝐶𝑝,𝑒𝑥 𝑑𝑇 (2) 1 In a thermogram, a first order phase transition can be seen by a peak in the heat flow. For polymers and other such chemical substances, glass phase transitions are also possible.3 This transition is an example of a second order phase transition where the substance remains the same phase but some other properties are changed. At the glass transition temperature (Tg), the polymer becomes much harder or softer depending on whether the sample is being cooled or heated.4 The heat capacity of a polymer is higher above the glass temperature and thus requires more energy to heat. Using the heat capacity, the change in entropy (S) can be calculated for the glass phase transition: 𝑇 𝐶𝑝 𝛥𝑆 = ∫𝑇 2 1 𝑇 𝑑𝑇 (3) In a thermogram, a glass phase transition can be seen where the graph turns from concave up to concave down (inflection point). The polymers to be tested in this experiment are isotactic polypropylene and amorphous polypropylene. Isotactic polypropylene has a structured arrangement of methyl groups along each stereo-center in the carbon backbone.1 This structured arrangement allows isotactic polypropylene to crystalize and melt in the suitable temperature range.4 The amorphous polymer on the other hand has a random arrangement of methyl groups and is not able to crystalize; it remains a rubbery texture. In order to find the degree of crystallinity in a sample of propylene, the enthalpy of fusion can be divided by the enthalpy of fusion of pure isotactic polypropylene. The information that can be obtained through differential scanning calorimetry, is often used to characterize materials.1 Polymers in particular are analyzed by DSC and the results can be used by manufacturers to test the quality of whatever product they are producing. It is important for the product to be of high quality to ensure the customer is receiving what they are ordering. DSC is also widely used in safety screening of building materials to ensure they possess the correct properties as well as in drug analysis.5 Experimental: Samples Analyzed: 1. Polypropylene, Isotactic [25085-53-4] Aldrich Repeating unit structure: [-CH2CH(CH3)-]n Average molecular weight: 250,000 g (determined by GPC) Density: 0.9 g/L Lot number: 02522BQ Catalog number: 18238-S 2. Polypropylene, amorphous [428175-1KG] Aldrich Repeating unit structure: [-C3H6-]n Average molecular weight: 14,000 g Density: 0.865 g/L Batch number: 057118-AH Instrument Used: DSC DSC Q200 differential scanning calorimeter Using tweezers, the reference pan was placed on the left rear cylindrical thermocouple. The PPC (isotactic polypropylene 9.5mg) sample was placed on the right thermocouple. The sample and reference were heated at a linear rate of ten degrees Celsius per minute using the custom prepared until the run was complete. Procedure was repeated to measure the PPA (amorphous polypropylene 9.3mg) sample. Results: The DSC analysis of amorphous polypropylene was carried out by heating at 10°C per minute and the resulting plot is displayed below in Figure 1. Figure 1: Graph of Heat Flow vs T for amorphous polypropylene Using the graph above, the following values can be calculated: ∆H = 17.97 J/g using equation 2 from above, this value was found by finding the area under the curve during the solid to liquid phase transition Tg = -13.87°C, this number refers to the temperature where the glass phase transition occurs which is found by finding the inflection point of the glass phase transition The same DSC analysis was carried out on the isotactic polypropylene and the resulting plot is displayed below. Figure 2: Graph of Heat Flow vs Temperature for isotactic polypropylene Using the Graph above, the following values can be calculated: ∆H = 86.97 J/g , this value is found by finding the area under the curve during the solid phase transition There is no glass phase transition for the isotactic sample The percent crystallinity in the amorphous sample can be found dividing the ∆H value for the amorphous by the ∆H of the isotactic sample. ∆Ham/∆Hiso = 0.2066 x 100 = 20.66% crystallinity In order to find the change in entropy involved in the glass phase transition, Cp/T must be plotted vs T and integrated using Tg - 20°C as T1 and Tg +20°C as T2 in equation 3. This graph is shown below in Figure 3. Cp/T vs T at ± 20 °C from Tg -0.5 -40 -30 -20 -10 -0.55 0 10 -0.6 Cp/T -0.65 -0.7 -0.75 -0.8 -0.85 -0.9 Temperature (C) -0.95 Figure 3: Graph of Cp/T vs T at ± 20°C from Tg From the graph above, ∆S can be calculated for the glass phase transition by using equation 3. ∆S = -28.16 J/g oC *was unable to find a value for this for amorphous polypropylene The change in entropy was plotted vs T to see how it varies with time. This graph can be seen in Figure 4 below. ∆S vs. T at ± 20 °C from Tg 0 -40 -35 -30 -25 -20 -15 -10 -5 0 5 10 -5 -10 ∆S -15 y = -0.6758x - 25.351 -20 R² = 0.9899 -25 -30 Temperature (C) -35 Figure 4: Graph of ∆S vs T at ± 20 °C from Tg Discussion: The typical heat of fusion (change in entropy) for isotactic polypropylene is 88 J/g and the obtained experimental value was 86.97 J/g.6 These numbers are within 2% error which is very good. This suggests that the DSC analysis that was carried out was accurate. The accepted value for Tg of amorphous polypropylene is -20 °C while the experimental value obtained was -13.87 °C.7 These values are close but not exactly the same. The reason that these numbers do not match is the presence of some amount of isotactic polypropylene in the amorphous sample. Amorphous polypropylene should not undergo a first degree phase change and melt completely because it cannot become fully crystalline due to its structure. Since it should not experience such phase transition, the thermogram should not display a peak in heat flow. The fact that there is a peak in the graph indicates the presence of some isotactic polypropylene. The label on the amorphous polypropylene suggests that the sample is pure but in reality, it is labelled incorrectly. In order to see how impure the sample of isotactic polypropylene is, the percent crystallinity was calculated to be 20.66%. This is a lot of impurity to not include in the label. This impurity changes the properties of the amorphous polypropylene and may cause the product to be ineffective for what how the customer wants to use it. In this case, a manufacturer could potentially lose money and have to recall their product that is improperly labeled. There are a few sources of error that can be associated with this experiment. One main source could be a resultant of the large amounts of data obtained and the analysis of that data. Another source of error could be involved with the machine itself and how it is calibrated. A third source of error would be associated with the preparation of the samples that are being tested, if they were not prepared exactly or with the correct weight reported, the data would not be accurate. One last source of error is involved with the integration of the heat flow peaks to find the heat of fusion. Inexact points were used to find the area underneath the curve using the program associated with the instrument. Repeating the experiment more than once would ensure the accuracy of the data obtained. The results obtained are relatively limited to the two particular samples that were tested but should be close to the values corresponding to another batch of amorphous polypropylene with 20 % crystallinity and another batch of isotactic polypropylene. The experiment was successful in determining the glass phase transition temperature of the amorphous sample as well as the heat of fusion in the amorphous sample. This analysis allowed the conclusion that the amorphous polypropylene sample was improperly labeled. Conclusion: An isotactic and amorphous sample of polypropylene were analyzed via Differential Scanning Calorimetry in order to determine the glass phase transition temperature of the amorphous sample and the heat of fusion of the isotactic sample. Through the presence of a peak in the thermogram of the amorphous sample, it became evident that the sample was not pure. In order to find how much isotactic polypropylene was present in the sample, the percent crystallinity was calculated. Since this amorphous sample was not pure, it was labeled wrong by the manufacturer Aldrich. This analysis is useful in determining the purity and properties of polymer products being manufactured. It is important for manufacturers to label their products properly or the customers will receive what they want. References: 1. Milosavljevic, Bratojub Chem 457 Experimental Physical Chemistry, Spring 2014 2. "Differential Scanning Calorimetry Analysis." Intertek. N.p., n.d. Web. 6 Mar. 2014. 3. "Differential Scanning Calorimetry." UC Davis Chem Wiki. Universtiy of California, n.d. Web. 6 Mar. 2014. 4. "Differential Scanning Calorimetry." Universtityof Southern Mississippi. Polymer Science Learning Center, 2005. Web. 5. Pungor, Erno (1995). A Practical Guide to Instrumental Analysis. Florida: Boca Raton. pp. 181–191. 6. “Typical engineering properties of Polypropyle.” INEOS Olefins and Polymers. February 2010 7. “Polymer Chemistry.” National Science Foundation's Division of Undergraduate Education. July, 2011