AP Chem Unit 12 HW Packet Name
advertisement
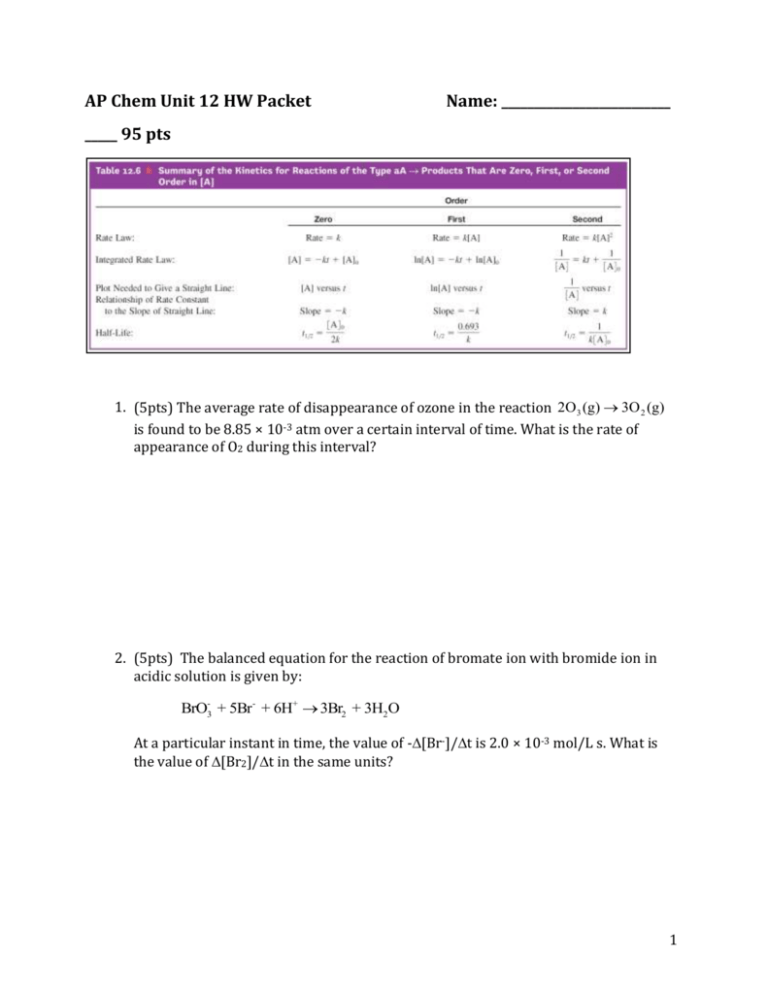
AP Chem Unit 12 HW Packet Name: __________________________ _____ 95 pts 1. (5pts) The average rate of disappearance of ozone in the reaction 2O 3 (g) 3O 2 (g) is found to be 8.85 × 10-3 atm over a certain interval of time. What is the rate of appearance of O2 during this interval? 2. (5pts) The balanced equation for the reaction of bromate ion with bromide ion in acidic solution is given by: BrO3- + 5Br- + 6H+ 3Br2 + 3H2O At a particular instant in time, the value of -[Br-]/t is 2.0 × 10-3 mol/L s. What is the value of [Br2]/t in the same units? 1 3. (10pts) For a reaction in which A and B react to form C, the following initial rate data were obtained: [A] [B] Initial Rate of Formation of C (mol/L) (mol/L) (mol/L·s) 0.10 0.10 1.00 0.10 0.20 4.00 0.20 0.20 8.00 What is the rate law? A) Rate = k[A][B] D) Rate = k[A]2[B]2 B) Rate = k[A]2[B] E) Rate = k[A]3 C) Rate = k[A][B]2 Justification: 2 4. (10pts) Tabulated below are initial rate data for the reaction 2Fe(CN)63– + 2I– 2Fe(CN)64– + I2 Initial Run [Fe(CN)63–]0 [I–]0 [Fe(CN)64–]0 [I2]0 Rate (M/s) 1 0.01 0.01 0.01 0.01 1 × 10–5 2 0.01 0.02 0.01 0.01 2 × 10–5 3 0.02 0.02 0.01 0.01 8 × 10–5 4 0.02 0.02 0.02 0.01 8 × 10–5 5 0.02 0.02 0.02 0.02 8 × 10–5 The experimental rate law is: A) I2 t B) I2 t C) I2 t D) I2 t E) I2 t = k[Fe(CN)63–]2[I–]2[Fe(CN)64–]2[I2] = k[Fe(CN)63–]2[I–][Fe(CN)64–][I2] = k[Fe(CN)63–)]2[I–] = k[Fe(CN)63–][I–]2 = k[Fe(CN)63–][I–] [Fe(CN)64–] Justification: 3 5. (10pts) The reaction of (CH3)3CBr with hydroxide ion proceeds with the formation of (CH3)3COH. (CH3)3CBr(aq) + OH–(aq) (CH3)3COH(aq) + Br–(aq) The following data were obtained at 55C. [(CH3)3CBr]0 [OH–]0 Inital Rate Exp. (mol/L) (mol/L) (mol/L) 1 0.10 0.10 1.0 10-3 2 0.20 0.10 2.0 10-3 3 0.10 0.20 1.0 10-3 4 0.30 0.20 ? What will the initial rate (in mol/L·s) be in Experiment 4? A) 3.0 × 10–3 B) 6.0 × 10–3 C) 9.0 × 10–3 D) 18 × 10–3 E) none of these Work: 6. (5pts) The reaction 2NO N2 + O2 has the following rate law: [ NO] 2k[NO]2 . t After a period of 2.9 × 103 s, the concentration of NO falls from an initial value of 2.8 10-3 mol/L to 2.0 10-3 mol/L. What is the rate constant, k? Work: 4 The following questions refer to the gas-phase decomposition of ethylene chloride. C2H5Cl products Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction: SHOW WORK. Time (s) ln [C2H5Cl] (M) 1.0 –1.625 2.0 –1.735 7. (5pts) What is the rate constant for this decomposition? A) 0.29/s B) 0.35/s C) 0.11/s D) 0.02/s E) 0.22/s 8. (5pts) What was the initial concentration of the ethylene chloride? A) 0.29 M B) 0.35 M C) 0.11 M D) 0.02 M E) 0.22 M 9. (5pts) What would the concentration be after 5.0 seconds? A) 0.13 M B) 0.08 M C) 0.02 M D) 0.19 M E) 0.12 M 10. (5pts) What is the time to half-life? A) 0.7 s B) 1.3 s C) 8.9 s D) 6.3 s E) 2.2 s 5 11. (5pts) Determine the molecularity of the following elementary reaction: O3 O2 + O. A) unimolecular B) bimolecular C) termolecular D) quadmolecular E) the molecularity cannot be determined Justification: 12. (5pts) The decomposition of ozone may occur through the two-step mechanism shown: step 1 O3 O2 + O step 2 O3 + O 2O2 The oxygen atom is considered to be a(n) A) reactant D) reaction intermediate B) product E) activated complex C) catalyst Justification: 6 13. (5pts) The reaction 2A + B C has the following proposed mechanism: Step 1: A + B equilibrium) D (fast Step 2: D+BE Step 3: E+AC+B If step 2 is the rate-determining step, then the rate of formation of C should equal: A) k[A] B) k[A]2[B] C) k[A]2[B]2 D) k[A][B] E) k[A][B]2 Justification: 14. (5pts) What would happen if the kinetic energy of the reactants was not enough to provide the needed activation energy? A) The products would be produced at a lower energy state. B) The rate of the reaction would tend to increase. C) The activated complex would convert into products. D) The reactants would continue to exist in their present form. E) The products would form at an unstable energy state. Justification: 7 15 (5pts) The reaction 2H2O2 2H2O + O2 has the following mechanism: H2O2 + I– H2O + IO– H2O2 + IO– H2O + O2 + I– The catalyst in the reaction is: A) H2O B) I– C) H2O2 D) IO– Justification: A reaction represented by the equation was studied at a specific temperature and the following data were collected: (Pressure is a direct result of concentration) 3O2 (g) 2O3 (g) time (seconds) Total pressure (atm) 0 1.000 46.89 0.9500 98.82 0.9033 137.9 0.8733 200.0 0.8333 286.9 0.7900 337.9 0.7700 511.3 0.7233 8 16. (10pts) What is the rate law for this reaction? 9









