Template for Electronic Submission to ACS Journals
advertisement

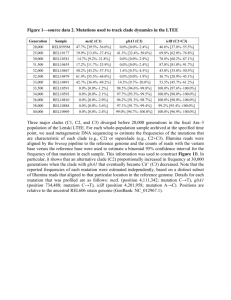
A Conserved Threonine Spring-loads Precursor for Intein Splicing Albert K. Dearden1, Brian Callahan2, Patrick Van Roey3, Zhong Li3, Utsav Kumar1, Marlene Belfort2,**, and Saroj K. Nayak1,** 1 Department of Physics, Applied Physics, and Astronomy, Rensselaer Polytechnic Institute, Troy, New York 12180, United States 2 Department of Biological Sciences, University at Albany, Albany, New York 12222, United States 3 Wadsworth Center, New York State Department of Health, Albany, New York 12201, United States ** Corresponding authors: nayaks@rpi.edu, mbelfort@albany.edu Supplemental Material Table S1. Gly-1 dihedral angles and their corresponding location in the Ramachandran plot. Values obtained for the various mutations. The first two mutations were derived experimentally whereas the remaining data are from computational work. Thr QM and Thr QM/MM refer to the full quantum mechanical calculation and the mixed quantum mechanical and molecular mechanical methods respectively, while Thr-S refers to the threonine calculation starting from the completed serine optimization. Region indicates the location in the Ramachandran plot to which the dihedral angles correspond, either allowed (A) or non-allowed (NA). Mutation ψ Region Thr69 Exp. -122.5 86.9 NA Ala Exp. -88.0 29.8 A Thr QM -134.2 74.7 NA Thr QM/MM -123.2 77.8 NA Thr-S -124.6 53.0 A Gly -99.4 54.4 A Ala -119.6 50.7 A Ser(1) -124.6 53.0 A Ser(2) -129.6 72.0 NA Val -116.9 51.9 A Cys -119.7 52.4 A Asp -117.6 51.9 A 1 Table S2. Computational Values for the Reaction Barriers. Computational values for the reaction barriers involving the native Thr69 and the T69A mutation. Each mutation is grouped with its corresponding allowed (A) or non-allowed (NA) region in the Ramachandran plot, its energy barrier, and charge values. HThr refers to the hydroxyl hydrogen of the threonine side chain, S Cys to the attacking sulfur of the cysteine side chain, OGly to the glycine oxygen receiving the hydrogen during the transition, OPro to the carbonyl oxygen atom of Pro-2, and NCys to the peptide nitrogen at the glycine-cysteine junction. ψ Region Barrier (kcal/mol) Charge (e) HThr SCys OGly OPro NCys -123.2 77.8 NA 14.8 0.52 -0.26 -0.67 -0.71 -0.64 -124.6 53.0 A 34.6 0.52 -0.37 -0.65 -0.70 -0.62 -119.6 50.7 A 24.9 N/A -0.51 -0.65 -0.67 -0.60 -123.2 77.8 NA 48.3 N/A -0.25 -0.77 -0.68 -0.60 Thr Ala Table S3. Oligonucleotides used in this study Gene/Purpose ID Sequence Site Feature DnaE / T69A 2845 mutation GTTCAGTAATCCGAGCTGCGTCTG ACCACCGCTTTTTAAC - C-term fragment DnaE / T69A 2846 mutation GTTAAAAAGCGGTGGTCAGACGCA GCTCGGATTACTGAAC N-term fragment DnaE / T69S 3146 mutation GATGGTTCAGTAATCCGAGCTAGC TCTGACCACCGCTTTTTAACCACC G - C-term fragment DnaE / T69S 3147 mutation CGGTGGTTAAAAAGCGGTGGTCAG AGCTAGCTCGGATTACTGAACCAT C - N-term fragment DnaE / T69A 3085 and T69S (pET45b) AAACACGTGCTGGTGCCGCGCGGC AGTCCGGATCCCTTTTGCCCTGGTT GCCTGTC PmlI LVPRG protease site, CPGC DnaE T69A and 3086 T69S (pET45b) AAACTCGAGTCACTGGACGTTAAA GCAGTTAGCTGCGATAGCGCC XhoI REV primer 2 Table S4. Data collection, phasing and refinement statistics Resolution (Å) 29.6-1.8 (1.86-1.8) Unique reflections 16636 Redundancy 2.56 (2.37) Completeness (%) 99.3 (98.2) Rmer (%) 0.034 (0.342) I/σ(I) 13.6 (2.5) Rcryst 0.226 Rfree 0.268 R.m.s.d. from ideality Bond lengths (Å) 0.005 Bond angles () 1.2 Dihedrals () 24.0 2 Average B-factors (Å ) Protein 37.0 Solvent 45.9 3 Figure S1. Comparison of reported CPGC loops with the wild type and T69A intein. Other than in the engineered DnaE inteins discussed here, a Cys-Pro-Gly-Cys peptide with a disulfide bond has been found in the PDB in only two structures: PDB entries 1JBQ (red) and 1M6Y (orange). Superposition of these four observations shows that the conformation of this loop in the native DnaE structure (3NZM, green) deviates more from the two unrelated observations than the loop in the T69A mutant (4GIG, cyan) and that the difference is the greatest in the conformation of Gly-1 (top right). Figure S2. Superposition of the CPGC loop in the native and T69A mutant of the DnaE intein. The Thr to Ala mutation, in addition to having removing the strain on the loop at Gly-1 allows for the insertion of a water molecule between the loop and Ala69 that results in a radically different hydrogen bonding pattern as discussed in the text. 4