ESID online Patient and Research database
advertisement
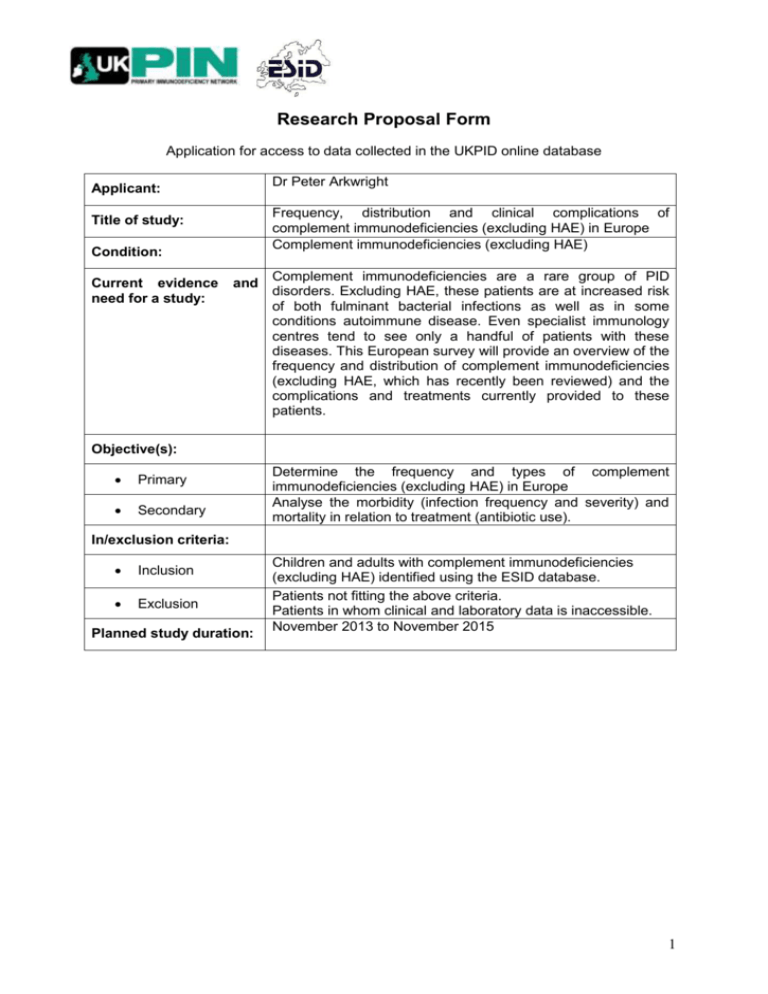
Research Proposal Form Application for access to data collected in the UKPID online database Dr Peter Arkwright Applicant: Frequency, distribution and clinical complications of complement immunodeficiencies (excluding HAE) in Europe Complement immunodeficiencies (excluding HAE) Title of study: Condition: Current evidence need for a study: and Complement immunodeficiencies are a rare group of PID disorders. Excluding HAE, these patients are at increased risk of both fulminant bacterial infections as well as in some conditions autoimmune disease. Even specialist immunology centres tend to see only a handful of patients with these diseases. This European survey will provide an overview of the frequency and distribution of complement immunodeficiencies (excluding HAE, which has recently been reviewed) and the complications and treatments currently provided to these patients. Objective(s): Primary Secondary Determine the frequency and types of complement immunodeficiencies (excluding HAE) in Europe Analyse the morbidity (infection frequency and severity) and mortality in relation to treatment (antibiotic use). In/exclusion criteria: Inclusion Exclusion Planned study duration: Children and adults with complement immunodeficiencies (excluding HAE) identified using the ESID database. Patients not fitting the above criteria. Patients in whom clinical and laboratory data is inaccessible. November 2013 to November 2015 1 1 It is important that the study concept is discussed with a statistician prior to initiating data extraction and analysis. Please add the following information: 1. Previous experience in research in primary immunodeficiencies (list 3 relevant publications) I am accredited by the GMC/RCPCH as a specialist in paediatric immunologist and have worked as a specialist leading the paediatric immunodeficiency service for Manchester and the North West of England for the last 11 years. I am also a senior lecturer in paediatric immunology at the University of Manchester and have over 100 peer-reviewed publications, many of which relate to PID. I am the lead investigator for Manchester for the ESID registry. A. Edgar JD, Buckland M, Guzman D, Conlon NP, Knerr V, Bangs C, Reiser V, Panahloo Z, Workman S, Slatter M, Gennery AR, Davies EG, Allwood Z, Arkwright PD, Helbert M, Longhurst HJ, Grigoriadou S, Devlin LA, Huissoon A, Krishna MT, Hackett S, Kumararatne DS, Condliffe AM, Baxendale H, Henderson K, Bethune C, Symons C, Wood P, Ford K, Patel S, Jain R, Jolles S, El-Shanawany T, Alachkar H, Herwadkar A, Sargur R, Shrimpton A, Hayman G, Abuzakouk M, Spickett G, Darroch CJ, Paulus S, Marshall SE, McDermott EM, Heath PT, Herriot R, Noorani S, Turner M, Khan S, Grimbacher B. The United Kingdom Primary Immune Deficiency (UKPID) Registry: Report of the first 4 years’ activity 20082012. Clin Exp Immunol 2013; Jul 11. B. Crawford G, Enders A, Gileadi U, Stankovic S, Zhang Q, Lambe T, Crockford TL, Lockstone HE, Freeman A, Arkwright PD, Smart JM, Ma CS, Tangye SG, Goodnow CC, Cerundolo V, Godfrey DI, Su HC, Randall KL, Cornall RC. DOCK8 is critical for the survival and function of NKT cells. Blood 2013122:2052-61. C. Jolles S, Williams P, Carne E, Mian H, Huissoon A, Wong G, Hackett S, Lortan J, Platts V, Longhurst H, Grigoriadou S, Dempster J, Deacock S, Kahn S, Darroch J, Simon C, Thomas M, Pavaladurai V, Alachkar H, Herwadker A, Abinun M, Arkwright P, Tarzi M, Helbert M, Bangs C, Pastacaldi C, Phillips C, Bennett H, El-Shanawany T. A UK National Audit of Hereditary and Acquired Angioedema. Clin Exp Immunol 2013 Jun 21. 2. A disclosure statement regarding potential conflicts of interest (such as financial affiliations with pharmaceutical companies). I declare no conflict of interests, financial or otherwise. 2 Evaluation criteria for registry research proposals (1 - 5 points each): Is the proposal within the scope of the UKPID Database? Does this proposal address an important problem? Are the conceptual or clinical framework, design, methods, and analyses adequately developed, and appropriate to the aims of the proposal? Is the study feasible? Is the proposal original and innovative? Are the investigators appropriately trained and suited to carry out this work? Does the scientific environment in which the work will be done contribute to the probability of success? One of the investigators should be a recognized specialist in the subject. 3