Mystery Powders Background
advertisement
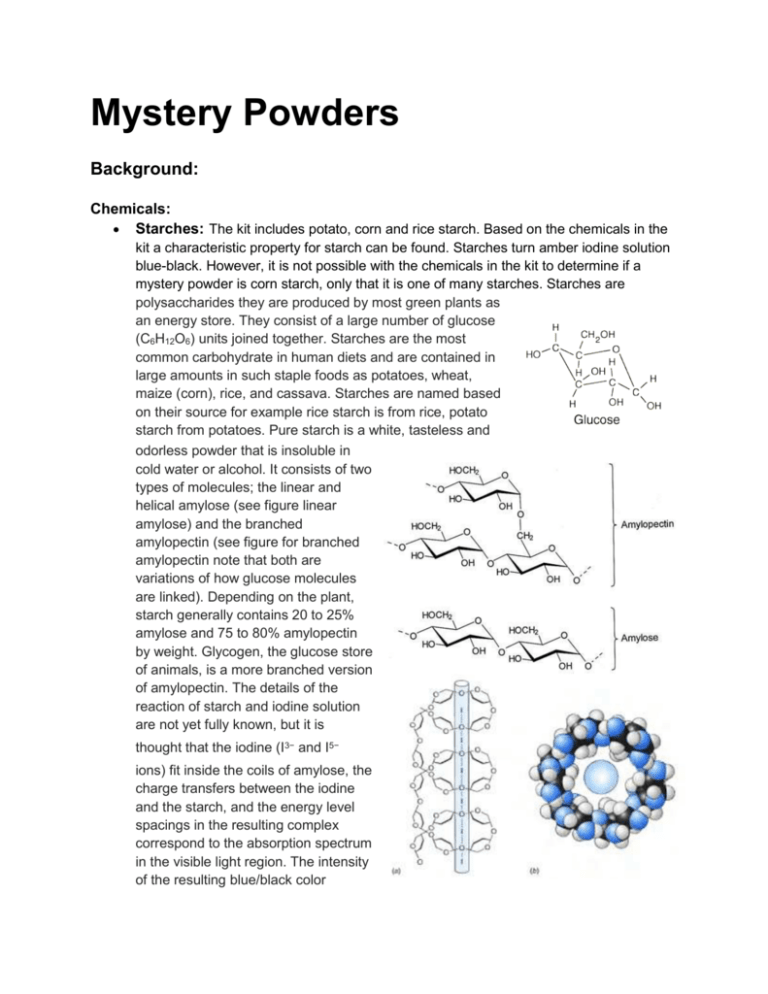
Mystery Powders Background: Chemicals: Starches: The kit includes potato, corn and rice starch. Based on the chemicals in the kit a characteristic property for starch can be found. Starches turn amber iodine solution blue-black. However, it is not possible with the chemicals in the kit to determine if a mystery powder is corn starch, only that it is one of many starches. Starches are polysaccharides they are produced by most green plants as an energy store. They consist of a large number of glucose (C6H12O6) units joined together. Starches are the most common carbohydrate in human diets and are contained in large amounts in such staple foods as potatoes, wheat, maize (corn), rice, and cassava. Starches are named based on their source for example rice starch is from rice, potato starch from potatoes. Pure starch is a white, tasteless and odorless powder that is insoluble in cold water or alcohol. It consists of two types of molecules; the linear and helical amylose (see figure linear amylose) and the branched amylopectin (see figure for branched amylopectin note that both are variations of how glucose molecules are linked). Depending on the plant, starch generally contains 20 to 25% amylose and 75 to 80% amylopectin by weight. Glycogen, the glucose store of animals, is a more branched version of amylopectin. The details of the reaction of starch and iodine solution are not yet fully known, but it is thought that the iodine (I3− and I5− ions) fit inside the coils of amylose, the charge transfers between the iodine and the starch, and the energy level spacings in the resulting complex correspond to the absorption spectrum in the visible light region. The intensity of the resulting blue/black color ● depends on the amount of amylose present. The diagram shows (a) Schematic structure of the starch iodine complex. The amylose chain forms a helix around I6 units and (b) a view down the starch helix showing iodine inside the helix. Carbonates: The kit includes sodium Na2CO3, magnesium MgCO3 and calcium CaCO3 carbonates as well as sodium hydrogen carbonate NaHCO3 The carbonate, CO3 part of the formula is common to all. The chemicals in the kit allow a characteristic property for carbonates to be found. Carbonates will fizz when vinegar/acetic acid is added. Thus it is possible to determine if a mystery powder is a carbonate but not to determine if it is sodium carbonate or sodium hydrogen carbonate or even if it is calcium carbonate. But if the powder fizzes with vinegar but does not dissolve in water then you would know it was either magnesium or calcium carbonate. The sodium carbonate and hydrogen carbonate are soluble in water and are used in the home as washing and baking soda. The magnesium and calcium carbonate are insoluble in water and are components of limestone. All carbonates and hydrogen carbonates react with acids such as acetic acid (vinegar) to produce carbon dioxide and water example Na2CO3(s) + 2 CH3COOH(aq) → 2 NaCH3COO(aq) + H2O(l) + CO2(g) Carbonates are also important in maintaining the ● pH of the blood. Several carbonates have been and still are important industrially for example: Soda ash (Na2CO3), or sodium carbonate, is used in the manufacture of glass, paper, rayon, soaps, and detergents. It is also used as a water softener, since carbonate can precipitate the calcium and magnesium ions present in "hard" water. Soda ash is also used to control pH (carbonate solutions neutralize acids, producing only carbon dioxide and water). Sodium carbonate is used in the chemical industry to synthesize many different sodium compounds, including sodium bicarbonate [common name for sodium hydrogen carbonate] (baking soda), sodium silicate (used in detergents), sodium tripolyphosphate (a detergent builder), sodium hydroxide (lye), sodium chromate and sodium dichromate (used in chrome plating), sodium aluminate (used in refining aluminum), and sodium cyanide (for electroplating). Limestone (CaCO3) is used in refining iron ore and manufacturing steel, making agricultural lime, making cement, in scrubbers that remove sulfur from flue gases, and the manufacture of soda ash. Sugars: The kit includes sucrose, fructose and glucose. Sucrose is the only sugar easily obtained. The sweetness property of sugars is not testable because taste in not an appropriate test, but students can observe that they are soluble in water. Sugars are carbohydrates and have the general formula of Cx(H2O)y . Sucrose is a disaccharide, made up of the two sugars glucose (C6H12O6) and fructose (C6H12O6) its formula is C12H22O11 when the two sugars are joined a molecule of water is split out. A characteristic property of sugars cannot be found using the chemicals in the kit so if a mystery powder is a sugar it only can be determined by identifying all the other mystery powders, i.e. by elimination. ● ● ● Salts: The kit includes sodium chloride (NaCl) (table salt) and Epsom salts (MgSO4.7H2O) (Magnesium sulphate). However, all the carbonates are salts as well. Like the sugars salts have a characteristic taste, they taste salty, which is not testable but the solubility can be observed. Thus a characteristic property of salts that are not carbonates cannot be found using the chemicals in the kit so if a mystery powder is a non carbonate salt it only can be determined by identifying all the other mystery powders, i.e. by elimination. Bases: The kit includes calcium hydroxide (Ca(OH)2) and magnesium hydroxide (Mg(OH)2). The OH group or part of the formula is common to all hydroxides and is common to all Arrhenius bases. NOTE the format of writing the chemical formula when stressing the acid base behaviour the OH group is kept together and follows the symbol of the metallic element. Bases when in solution feel slippery to the touch and will change indicators like litmus from red to blue and bromothymol blue solution from green to blue. Bases also turn red cabbage juice from magenta to blue/green as seen in the photo above. Acids: The kit includes acetic acid (CH3COOH) (vinegar), which is one of the testing reagents. The two white powders that are acids are acetylsalicylic acid (aspirin, ASA, C8H7O2COOH) and one of benzoic acid (C6H5COOH) or oxalic acid ((COOH)2). The COOH group or part of the formula is common to acids that are organic, i.e. carbon based. Inorganic acids such as HCl (hydrochloric acid), HNO3 (nitric acid) and H2SO4 (sulphuric acid) are characterized by the symbol of hydrogen, H at the beginning of the formula. NOTE the format of writing the chemical formulas of organic acids when stressing the acid base properties the COOH group is kept together and follows the symbols of carbon, hydrogen and any other elements. For inorganic acids the H is at the beginning of the formula followed by the symbol of a non-metallic element and then maybe symbols of oxygen. NOTE those reading this unfamiliar with “chemist idiosyncrasies” might be unaware that chemists write the formula of a substance in different ways depending on the context the chemist is using. The proportion of the elements shown by the formula never changes but the way the formula is displayed may change. For example benzoic acid. If we are only interested in the elements and their proportions we would use C7H6O2. If we are interested in its acid base properties we would display it as C6H5COOH and if we were interested in how it interacts with other substances we would use a formula which emphasises its bonds, see the graphic structure to the right. All of these representations of the benzoic acid molecule are equivalent in terms of elements involved and their proportions but the display has been adjusted to show more about how we believe that the elements’ atoms are attached to each other. Which one a chemist will use depends on the context. Acids in solution taste bitter like vinegar and change indicators like litmus from blue to red, and bromothymol blue solution from green to yellow. Acids also turn red cabbage juice from magenta to red as shown in the photo above. Reactions: Carbonates with Acid (carbonates plus an acid produces a salt, water and carbon dioxide) Na2CO3(s) + 2 CH3COOH(aq) → 2 NaCH3COO(aq) + H2O(l) + CO2(g) MgCO3(s) + 2 CH3COOH(aq) → Mg(CH3COO)2(aq) + H2O(l) + CO2(g) Acids and Bases (base plus acid produces a salt and water) Ca(OH)2(aq) + C8H7O2COOH(aq) → Ca(C8H7O2COO)2(s) + 2 H2O(l) A Model for Indicators (General Formula HIn to be an indicator HIn has one colour and In1- a different colour) Ca(OH)2(aq) + HIn(aq) → Ca(In)2(aq) + 2 H2O(l) this equation is usually only shown in ionic format OH1-(aq) + HIn(aq) → In1-(aq) + H2O(l) the equivalent for acid is H1+(aq) + In1-(aq) → HIn(aq) the combination of these two reactions is OH1-(aq) + 2 HIn(aq) ↔ 2 In1-(aq) + H2O(l) + H1+(aq) the equation is further simplified by combining the H2O(l) and H1+(aq) to form H3O1+(aq) so the model becomes OH1-(aq) + 2 HIn(aq) ↔ 2 In1-(aq) + H3O1+(aq) So to explain indicator colour changes we first consider adding some base represented by the OH1- which reacts with HIn to form In1- this changes the colour from the HIn colour to the In1- colour. Similarly adding some acid represented by the H3O1+ it reacts with the In1- to form the HIn so the colour changes from the In1- colour to the HIn colour. the intermediary colours which are combinations of the two different colours are caused by situations where you have some of both HIn and In1- in solution, the base or acid added has not totally reacted with the indicator Challenge: Your challenge, should you accept it, and you will, is to find the identity of 4 mystery powders. You have the following materials that you can use. You will have to first discover ways to test for various types of powders and then use the tests you find to test the mystery powders. Plan your approach you must be able to back your choice of identity for each of the four powders and you only get ONE chance to identify them. Materials (per station for 5 students) ● ● ● ● ● ● ● ● 1 - Microscope/Magnifying glass stir sticks (a measure is 1 cm of powder on the stir stick) 5 - artist palettes (4-8 depressions - Dollar Store) 17 - small pill containers (5 dram with lids) 1 - spray bottle filled with water for rinsing 1 - large bucket for rinsing 1 - tarp to cover table for easy clean up powders (suggestions, put into small pill containers, label with common and chemical name) ○ Potato Starch (grocery store) ○ Corn Starch (grocery store) ○ Rice Starch (grocery store) ○ Flour (grocery store) ○ Sodium Hydrogen Carbonate (Baking Soda - grocery store) ○ Sodium Carbonate (Washing Soda - grocery store) ○ Magnesium Carbonate (Limestone Screenings - landscaping store) ○ Calcium carbonate (antacid pills - pharmacy) ○ Sucrose (White Sugar - grocery store) ○ Fructose (available online) ○ Glucose (available online) ○ Sodium chloride (salt - grocery store) ○ Magnesium sulphate (Epsom salts - pharmacy) ○ Calcium hydroxide (hydrated lime - building supply store ) ○ ○ ○ ● Magnesium hydroxide (Milk of Magnesia - pharmacy) Benzoic acid (available online) Oxalic acid (teak cleaner - marine store) reagents ○ Vinegar (acetic acid - grocery store) ○ Water (tap) ○ Iodine solution (tincture of iodine - pharmacy) ○ Red cabbage juice (grocery store) To do: ● ● ● ● ● Look at a few particles of powder on a microscope slide or on black paper with a magnifying glass. Use ONE measure to squish between your thumb and finger. Use only ONE measure of the powders and ONE or TWO drops of the reagents in a depression of the artist pallet at one time. Use the stir stick to mix powder and reagent after first describing what happens when added. After using, rinse stir sticks and artist pallet into large bucket provided for that purpose. Dry with paper towels. DO NOT MIX THE POWDERS AT THIS TIME, UNTIL YOUR TEACHER TELLS YOU, YOU SHOULD MIX. Properties to Observe: 1. Squish powder between thumb and finger. (describe sounds and feelings use words like squeaky, no sound, smooth, sharp, rough, lumpy) 2. Add drop or two of "tincture of iodine" (describe colour changes, if any) 3. Add drop or two of vinegar (acetic acid) (describes changes, if any) 4. Add drop or two of red cabbage juice (describe colour change, if any) 5. Add drop or two of water (use words like; no change, wets, schlerin, powder completely, partially, half, disappears, 6. Describe shape and appearance as viewed under the microscope or magnifying glass (use words like; cube, sphere, needles, shiny, dull)







