Lab4 Photoelectric effect - the Advanced Physics Lab Wiki
advertisement

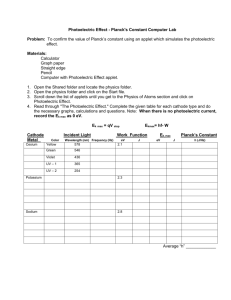
Andrews University Department of Physics The Photoelectric Effect: Determining Planck’s Constant A Lab Report Presented in Partial Fulfillment Of the Requirements of the Course PHYS377: Advanced Lab 1 Erik Vyhmeister & Jeremy Thomas Abstract This experiment used the photoelectric effect and specific apparatus to determine the value of Planck’s constant. A mercury lamp was used as the light source, and the energy of ejected electrons was determined by the stopping potential created by their moving to the anode. The value of Planck’s constant was determined to be within 0.25% of the accepted value, with a maximum uncertainty of +/- 5%. Introduction The purpose of this experiment is to determine Planck’s constant, h by using the photoelectric effect. The photoelectric effect occurs when photons incident on a material (typically a metal) impart enough energy to the highest-level electron, causing it to be ejected. The energy of the electron is modeled by the equation 𝐾𝑒𝑙𝑒𝑐𝑡𝑟𝑜𝑛 = ℎ𝑓 − 𝑊0 (1) Where h is Planck’s constant, f is the frequency of the photon, and W0 is the work function – the amount of energy it takes to get the electron out of its potential well. Electrons with energy K Photons Metal Figure 1. A visual demonstration of what the photoelectric effect looks like in action. In the experiment, we measured the voltage needed to stop outgoing electrons for different specific frequencies of light, thereby determining their energy in eV. From this, we will be able to determine the value of h by rearranging (1). ℎ 𝑉 = 𝑒∗𝑓− 𝑊0 𝑒 (2) Since the stopping potential depends only on f, the slope should be h/e, and with a known value of e we can determine h, Planck’s constant, which is crucial to much of quantum theory. Description of Experiment Equipment used: PASCO AP-9368 (h/e apparatus) Green, yellow filters Voltmeter The PASCO apparatus consists of a mercury lamp, which produces very specific frequencies of light. The light is then passed through a diffraction grating to separate out the different colors (frequencies). Mercury Lamp Diffraction Grating Target – cathode, anode Voltmeter Figure 2. A picture of the equipment used. The mercury lamp emits 6 distinct frequencies of light within the visible range. For the green and yellow bands, the green and yellow filters are needed (respectively) to ensure that no other higher energy light sneaks in. First, the lamp has to be warmed up. This takes about five minutes. We then connected the voltmeter to the detector apparatus. To gather data, we turned the light off (again, to prevent multiple frequencies of light being incident on the cathode), and positioned the apparatus such that only one color of light was incident on the detector. We then recorded the stopping voltage as well as the frequency of the incident light, which is known for mercury, and recorded in Table 1. After every measurement, it is necessary to clear the anode of its residual charge to get a clear reading. This is done by pressing a button on the side of the detector. Figure 3 explains the inner workings of the detector. Color Yellow Green Blue-green (weak) Blue Violet Ultraviolet Frequency (1014 Hz) 5.18672 5.48996 6.09830 6.87858 7.40858 8.20264 Table 1. Frequencies of light emitted by mercury. Figure 3 – A diagram of what is happening inside the detector. Data & Analysis Because of how little variance there was between the two readings, it was determined that more data was not going to enhance the accuracy or precision of the experiment, so only two trials were made. (color) Frequency Volts (V) Yellow 5.19E+14 0.648 Green 5.49E+14 0.778 Blue-green 6.10E+14 1.103 Blue 6.88E+14 1.378 Violet 7.41E+14 1.592 Ultraviolet 8.20E+14 1.905 trial2 Yellow 5.19E+14 0.651 Green 5.49E+14 0.778 Blue-green 6.10E+14 1.109 Blue 6.88E+14 1.382 Violet 7.41E+14 1.592 Ultraviolet 8.20E+14 1.905 Table 2. Frequency of incident light compared with stopping potential (V). The above data was taken and placed in Graphical analysis, plotting frequency vs. Voltage. The following graph resulted: Figure 4. Graph of frequency vs voltage for our data. Gives the slope as 4.146E-15. Recalling equation (2), the slope should be equal to h/e. Using the accepted values for h and e, h/e should be 4.13567, which is only 0.25% different from the obtained value. The measured value of h was 6.636E-34, compared to 6.626E-34. According to the PASCO website, the work function of the photodiode (cathode) is 1.36 +/- 0.08 eV. Our measured W0 is 1.477 eV, which falls slightly outside that range. For more details on the exact calculations, see the attached Excel file. Error Analysis Because of the cleanness of the data, the only realistic source of error in this experiment is the voltmeter. For the voltmeter we were using, the Extech MN35, in the range we were using it the error was +/- 0.5%. Using only this error, the uncertainty in the measured value of h was as follows: Absolute error min value h 6.54E-34 1.339% max value h 6.74E-34 1.742% Table 3. Using error only from the multi-meter. However, if one also incorporates the error inherent in the use of linear regression, the uncertainty is somewhat larger. Absolute error min value h 6.30E-34 4.954% max value h 6.98E-34 5.357% Table 4. Using error from linear regression. Regardless of which method for the calculation of uncertainty, the median remains in the same place, at 6.636E34, and both sets of error bounds contain the actual value of Planck’s constant. The uncertainty for the work function of 1.477 eV was found to be 0.141, which is wide enough that it incorporates PASCO’s given value of 1.36 +/- 0.08 eV. Conclusion This experiment used the photoelectric effect and specific apparatus to determine the value of Planck’s constant. A mercury lamp was used as the light source, and the energy of ejected electrons was determined by the stopping potential created by their moving to the anode. The value of Planck’s constant was determined to be within 0.25% of the accepted value, with a maximum uncertainty of +/- 5%. We demonstrated that the photoelectric effect is both real and measurable. In order to improve both accuracy and precision of the lab, using more elements as sources of light would have been useful – it would have given more data points, and reduced the reliance on a single lamp.