Physics 270
advertisement

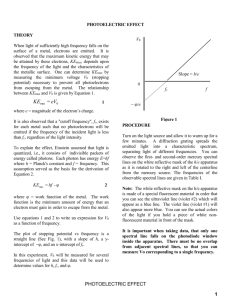
Laboratory #7 The Photoelectric Effect The photoelectric Effect plays a crucial role in our understanding of the behavior of light. In this laboratory you will investigate the dependence of stopping voltage on the intensity of the light and on the frequency (wavelength) of the light. Apparatus Pasco Scientific h/e Apparatus, Voltmeter, Mercury Source, neutral density filter, and green and yellow filters. Part A The dependence of stopping voltage on the intensity of the light source 1. Set up the equipment as shown in the diagram below. Apply power to the h/e apparatus. 2. Focus the light from the mercury vapor light source onto the slot in the white reflective mask on the h/e apparatus. For this part select one of the colors, say the first bright blue line. 3. Tilt the light shield of the apparatus out of the way to reveal the white photodiode mask inside the apparatus. Align the system by rotating the h/e apparatus on its support base so that the same color light that falls on the opening of the light screen falls on the window in the photodiode mask with no overlap of color from other spectral bands. Return the light shield to its closed position. Ask the instructor for help with this part. 4. Place the neutral density filter over the slit with the 100% transmission in position first. 5. Check the polarity of the leads from the digital voltmeter, and connect them to the output terminals of the same polarity on the h/e apparatus. Adjust the voltmeter for DC and the 20 volt scale (3 digits). 6. For the one color selected, measure the stopping voltage with the voltmeter. Do this by carefully pressing the reset button on the apparatus and record the voltmeter reading when it reached its final stable value. 7. Repeat the measurement for intensities of 80%, 60%, 40%, and 20% and record the results in the data table. 8. When you are finished, remove the neutral density filter. Analysis for Part A What can you conclude about the dependence of stopping voltage on intensity? Hint: One way to answer this question is to determine the % difference between the 100% intensity level and each of the other intensity levels. Physics 200 Laboratory #7 Page 1 of 2 Part B The dependence of stopping voltage frequency; Determining Planck’s constant 1. You can see five colors in two orders of the mercury light spectrum. Adjust the h/e apparatus carefully so that only one color starting with yellow from the first order falls on the opening of the mask of the photodiode. 2. Tilt the light shield of the apparatus out of the way to reveal the white photodiode mask inside the apparatus as you did in step 3 of Part A. Align the system by rotating the h/e apparatus on its support base so that the same color light that falls on the opening of the light screen falls on the window in the photodiode mask with no overlap of color from other spectral bands. Return the light shield to its closed position. Again. you may ask the instructor for help with this part. 3. For each color in the first order, measure the stopping voltage with the voltmeter. Use the yellow and green colored filters on the reflective mask of the apparatus when you employ the yellow and green spectral lines. These filters serve to eliminate higher orders of higher frequency light that overlap the visible light. 4. Each group should repeat this process for the light in the second order spectrum Analysis for Part B Determine the best value for h/e by performing a Trendline analysis on your data. A typical Excel spreadsheet is located in the folder for Laboratory #7. Note that the intercept would ordinarily be the work function W. In this case however, we do not have a single pure element for a cathode so the value will not be the work function for a particular element. The slope nevertheless should be equal to h/e. eVstopping hf W Vstopping h W f e e Note on units: If we multiply the stopping voltage by the charge on the electron, we would get the maximum kinetic energy of the electrons in joules. In atomic physics, it is convenient to use a new energy unit, the electron-volt. The electron-volt is the energy acquired by an electron when accelerated through a voltage difference of one volt. 1electronVolt 1.6 x1019 joules Since we are plotting the stopping voltage on the vertical axis and the frequency on the horizontal axis, the slope will be h/e. We may also interpret the results as determining Planck’s constant in units of electronVolt*seconds. We abbreviate this as eV*s. If you wish you may find Planck’s constant in joule*seconds by multiplying your result by the charge on the electron. 1. Include a copy of your Trendline analysis in your report 2. Compare your answer to the accepted value: 4.136 x 10-15 eVs It would be appropriate to find the percent difference. 3. Comment on the accuracy of your result. Lab Report Due: One week from today. Physics 200 Laboratory #7 Page 2 of 2