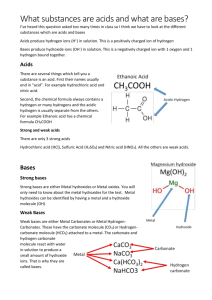
Acids and bases are everywhere!
advertisement

1/11/12 Acids and Bases I: Webquest On Your Desk: This packet Pencil Calculator Expectations: Students will work quietly and respectfully. Students will raise their hands before speaking Students will not leave their seats without permission Teacher will respect and encourage all questions Teacher will enforce all rules and expectations Introduction Acids and bases are everywhere! You use chemicals all the time! The deodorant, shampoo, face wash, etc. that you use are all made of chemicals, and they act differently from one another. You wouldn’t use vinegar to wash your hair, or laundry detergent in the dishwasher. Would you use toothpaste to flavor a cake? Would you use toilet bowl cleaner to perm your hair? Would you use lemonade as a replacement for chicken broth? When you have heartburn, would you take TUMS or drink orange juice? Come up with your own Ridiculous Substitution here! ________________________________________________________ WHY, though, are all these household compounds so different from one another? Can we learn enough about their chemistry to understand why some household materials act the way that they do? In today’s activity, we will learn that various household chemicals have different pHs. What is pH? The power of an acid or base is measured with the pH scale. pH means “the power of hydrogen”. The pH tells you how many hydrogen ions are in a solution. This affects a solution in many ways – from how it feels and tastes to how it will react chemically. Take a moment and remember what kind of ion hydrogen makes. Look at the periodic table if you need to! What ion does hydrogen form? __________________ The pH scale goes from 1-14. Water is neutral, and has a pH of 7. Acids have a pH below 7, and bases have a pH above 7. Each value of pH means the concentration of H+ changes by a power of 10. This means that a solution with a pH of 6 has 10 times more H+ ions in it than a solution with a pH of 7. As the concentration of H+ ions increases, the concentration of OH- ions decreases, and vice versa. At pH 7, the concentrations of H+ and OH- are equal. LJC 2011. pH graphic from http://www.chymist.com/history4.htm. Cabbage Lab from York/Phelps, BDHS, 2010 Questions 1. What does pH measure? _____________________________________________________________________________________ 2. The pH scale goes from ___________ to ______________. 3. A neutral solution has a pH of ______. An ________________ has a pH below 7, and a ______________ has a pH above 7. 4. Water has a pH of ______________ and is therefore considered ___________________________________. 5. A solution with a pH of 2 has ten times more hydrogen ions in it than a solution with a pH of _______. Reference Guide: Examples: For thousands of years people have known that lemon juice and many other foods taste sour. However, it was not until a few hundred years ago that it was discovered why these things taste sour - because they are all acids. The term acid, in fact, comes from the Latin term acere, which means "sour". Some substances, like battery acid, are extremely powerful and can burn through metal. Other acids, such as rainwater, are fairly weak and harmless to humans. Many people mistakenly believe that the opposite of acid is harmless to humans. However, this is not the case. The opposite of an acid is called a base (or alkali). Some bases, such as baking soda, are quite weak, while strong bases such as oven cleaner can easily burn human skin. Definitions: Acids are substances that produce hydrogen atoms (H) when dissolved into water. Bases are substances that produce molecules made of one hydrogen atom and one oxygen atom (HO) when combined with water. When an acid and a base are combined together they neutralize or cancel each other out. An acid produces an (H) molecule and a base produces an (HO) molecule that combine into H2O, or pure water. Characteristics: Acids and bases have certain characteristics. Acids tend to feel sticky when touched. They also tend to taste sour. (NEVER TASTE ANYTHING IN A SCIENCE LAB UNLESS INSTRUCTED TO DO SO!) Bases feel soapy when touched, and tend to taste bitter. Bases actually feel soapy because they dissolve a small amount of your skin when touched! Because strong bases can be very dangerous when consumed, many scientists believe that the bitter taste evolved as a natural defense mechanism for humans. Both acids and bases can burn your skin, and both can conduct electricity. Now that you have the basics of acids and bases, complete the webquest listed under Mr. Astor’s file cabinet on collegeready11.org. 1. What is an acid? What is a base? 2. How do scientists measure the strength of acids and bases? Describe this scale. 3. Describe the physical properties such as taste and feel of acids and bases. 4. Give 3 examples of acids and 3 examples of bases from the pH Scale on the site. 5. How do you neutralize an acid? A base? 6. Name a base that you could use to neutralize an acid. 7. What is an acid base indicator? Give an example used during the video. Reflection Questions 1. What did you already know about acids and bases before you began the webquest? 2. What were two things that you learned about acids and bases from the webquest? 3. What is one question you still have about acids and bases?