path 1281 to 1290 [10-4
advertisement

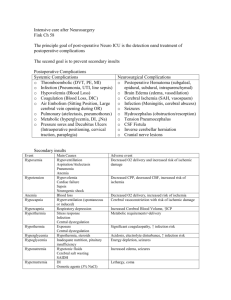
path 1281 to 1290 Reaction of Neurons to Injury Acute neuronal injury (red neurons) – spectrum of changes that accompany acute CNS hypoxia/ischemia or other acute insults and reflect cell death, either necrosis or apoptosis o 12-24 hours after irreversible hypoxic/ischemic insult, cell bodies shrink, there is pyknosis of nucleus, disappearance of nucleolus, and loss of Nissl substance, with intense eosinophilia of cytoplasm Subacute and chronic neuronal injury (degeneration) – neuronal death occurring as result of progressive disease process of some duration, as seen in slowly evolving neurologic disease such as amyotrophic lateral sclerosis (ALS) o Characteristic histologic feature is cell loss, often selectively involving functionally related groups of neurons , and reactive gliosis o Reactive glial changes is best indicator of pathologic process o For many disease, cell loss is due to apoptosis o Neuronal trans-synaptic degeneration seen when there is destructive process that interrupts majority of afferent input to group of neurons Axonal reaction – reaction within cell body that attends regeneration of axon o Best seen in anterior horn cells of spinal cord when motor axons cut or seriously damaged o Increased protein synthesis associated with axonal sprouting, reflected in enlargement and rounding up of cell body, peripheral displacement of nucleus, enlargement of nucleolus, and dispersion of Nissl substance from center to periphery of cell (central chromatolysis) Neuronal damage – may be associated with subcellular alterations in neuronal organelles and cytoskeleton o Neuronal inclusions may occur as manifestation of aging, when there are intracytoplasmic accumulations of complex lipids (lipofuscin), proteins, or carbohydrates o Abnormal cytoplasmic deposition of inclusions occurs in genetically determined disorders of metabolism in which substrates or intermediates accumulate o Viral infection can lead to abnormal intranuclear inclusions, as seen in rabies (Negri body), or both nucleus and cytoplasm as in cytomegalovirus (CMV) infection Some degenerative disease of CNS associated with neuronal intracytoplasmic inclusions, such as neurofibrillary tangles of Alzheimer’s disease and Lewy bodies of Parkinson’s disease o Others cause abnormal vacuolization of perikaryon and neuronal cell processes in neuropil (CreutzfeldtJakob disease) – these aggregates highly resistant to degradation, contain proteins with altered conformation, and may result from mutations that affect protein folding, ubiquitination, and intracellular trafficking – also called proteinopathies o Visible aggregates in many diseases not from basis of cellular injuries but from small multimers of proteins (oligomers) Reaction of Astrocytes to Injury Processes contain GFAP (glial fibrillary acidic protein), a cell type-specific intermediate filament Act as metabolic buffers and detoxifiers within brain Through foot processes, which surround capillaries or extend to subpial and subependymal zones, they contribute to barrier functions for macromolecule transport in blood, CSF, or brain Gliosis (astrogliosis) – most important histopathologic indicator of CNS injury – characterized by hypertrophy and hyperplasia o Nuclei become vesicular and develop prominent nucleoli o Cytoplasm expands to bright pink irregular swath around eccentric nucleus, from which emerge numerous processes o These cells called gemistocytic astrocytes When directly injured, astrocytes react with cytoplasmic swelling o Seen in acute insults that cause cell’s ATP-dependent ion channels to fail o Occurs in hypoxia, hypoglycemia, and toxic injuries Type II Alzheimer’s astrocytes – gray-matter cells with large nucleus, pale-staining central chromatin, intranuclear glycogen droplet, and prominent nuclear membrane and nucleolus o Seen more often in individuals with long-standing hyperammonemia due to chronic liver disease, Wilson disease, or hereditary metabolic disorders of urea cycle Rosenthal fibers – thick, elongated, brightly eosinophilic, somewhat irregular structures that occur within astrocytic processes and contain 2 heat-shock proteins (αB-crystallin and hsp27) as well as ubiquitin o Typically found in regions of long-standing gliosis o Characteristic of pilocytic astrocytoma (glial tumor) Alexander disease – leukodystrophy associated with mutations in gene encoding GFAP – has abundant Rosenthal fibers in periventricular, perivascular, and subpial locations Corpora amylacea (polyglucosan bodies) – round, faintly basophilic, PAS-positive, concentrically lamellated structures located wherever there are astrocytic end processes, especially in subpial and perivascular zones o Also contain heat-shock proteins and ubiquitin o Occur in increasing numbers with advancing age Lafora bodies – seen in cytoplasm of neurons, hepatocytes, myocytes, and other cells – occurs during myoclonic epilepsy – very similar to polyglucosan bodies Reactions of Other Glial Cells to Injury Oligodendrocytes and ependymal do not participate in active response to CNS injury and show more limited repertoire of reactions Oligodendroglial cytoplasmic processes wrap around exons and form myelin o Injury or apoptosis of oligodendrocytes is a feature of acquired demyelinating disorders and leukodystrophies o Nuclei may harbor viral inclusions in progressive multifocal leukoencephalopathy o Multiple system atrophy (MSA) – oligodendrocytes have glial cytoplasmic inclusions primarily composed of α-synuclein Ependymal cells do not have specific patterns of reaction o Disruption of ependymal lining is paired with proliferation of subependymal astrocytes to produce small irregularities on ventricular surfaces (ependymal granulations) o Certain infections agents, particularly CMV, may produce extensive ependymal injury with viral inclusions in ependymal cells Microglia – respond to injury by proliferating, developing elongated nuclei (rod cells) as in neurosyphilis, forming aggregates about small foci of tissue necrosis (microglial nodules), or congregating around cell bodies of dying neurons (neuronophagia) o Also recruit blood-derived macrophages (which are principal phagocytic cells in inflammatory foci) Cerebral Edema Brain parenchymal edema can be o Vasogenic edema – caused by BBB disruption and increased vascular permeability, allowing fluid to shift from intravascular compartment to intercellular spaces of brain Paucity of lymphatics greatly impairs resorption of excess extracellular fluid Edema may be localized or generalized o Cytotoxic edema – increase in intracellular fluid secondary to neuronal, glial, or endothelial cell membrane injury, as might be encountered in someone with generalized hypoxic/ischemic insult or metabolic damage Conditions associated with generalized edema often have elements of both types of edema Interstitial edema (hydrocephalic edema) occurs especially around lateral ventricles when increase in intravascular pressure causes abnormal flow of fluid from intraventricular CSF across ependymal lining to periventricular white matter In generalized edema, gyri are flattened, sulci are narrowed, and ventricular cavities are compressed – as brain expands, herniation may occur Hydrocephalus Most cases occur as consequence of impaired flow and resorption of CSF, but rarely overproduction of CSF can cause this Increased volume of CSF within ventricles expands them and can elevate intracranial pressure Hydrocephalus developing after infancy is associated with expansion of ventricles and increased intracranial pressure, without change in head circumference If only portion of ventricular system is enlarged because of excess CSF (as may occur because of a mass in the third ventricle), pattern called noncommunicating hydrocephalus Communicating hydrocephalus – enlargement of entire ventricular system Hydrocephalus ex vacuo – dilation of ventricular system with compensatory increase in CSF volume secondary to loss of brain parenchyma Raised Intracranial Pressure and Herniation When volume of brain increases beyond limit permitted by compression of veins and displacement of CSF, pressure within skull will increase Most cases associated with mass effect, either diffuse (as in generalized brain edema) or focal (as with tumors, abscesses, or hemorrhages) Elevated intracranial pressure can reduce perfusion of brain, further exacerbating cerebral edema Because cranial vault divided by rigid dural folds (falx and tentorium), localized expansion of brain may cause it to be displaced in relation to these partitions If expansion is sufficiently severe, herniation syndrome may occur o Subfalcine (cingulate) herniation – occurs when unilateral or asymmetric expansion of cerebral hemisphere displaces cingulate gyrus under falx cerebri – may lead to compression of branches of anterior cerebral artery o Transtentorial (uncinate, mesial temporal) herniation – occurs when medial aspect of temporal lobe is compressed against free margin of tentorium With increasing displacement of temporal lobe CN III is compromised, resulting in pupillary dilation and impairment of ocular movements on side of lesion Posterior cerebral artery may also be compressed, resulting in ischemic injury to primary visual cortex When extent of herniatin is large enough, contralateral cerebral peduncle may be compressed, resulting in hemiparesis ipsilateral to side of herniation (Kernohan’s notch formed in peduncle) Progression of transtentorial herniation often accompanied by hemorrhagic lesions in midbrain and pons (secondary brainstem or Duret hemorrhages) Linear or flame-shaped lesions Usually occur in midline and paramedian regions Due to distortion or tearing of penetrating veins and arteries supplying upper brainstem o Tonsillar herniation – displacement of cerebellar tonsils through foramen magnum Causes brainstem compression and compromises vital respiratory and cardiac centers in medulla oblongata Malformations and Developmental Diseases Neural tube defects – collectively account for most CNS malformations – can be caused by failure to close or spontaneous reopening of the area o Encephalocele – diverticulum of malformed CNS tissue extending through defect in cranium – most often occurs in occipital region or in posterior fossa o Spina bifida – also called spinal dysraphism Myelomeningocele (meningomyelocele) – CNS tissue projecting through defect Occur most commonly in lumbosacral region and patients have motor and sensory dysfunctions in lower extremities and/or disturbances in bowel and bladder control from disruption of the cord as well as superimposed infection that extends from thin, overlying skin Meningocele – only meningeal extrusion Monozygotic twins more likely to have a form of spina bifida Genetic factors usually have to do with folic acid enzymes o Anencephaly – absence of brain and calvarium Forebrain development disrupted at approximately 28 days of gestation and all that remains in its place is area cerebrovasculosa (flattened remnant of disorganized brain tissue with admixed ependymal, choroid plexus, and meningothelial cells Forebrain Anomalies o Megalencephaly – abnormally large volume of brain o Microencephaly – abnormally small volume of brain Can occur from chromosome abnormalities, fetal alcohol syndrome, and HIV-1 infection acquired in utero Could be caused by reduction in number of neurons that reach neocortex, leading to simplification of gyral folding Neuronal number determined by fraction of proliferating cells that undergo transition into migrating cells with each cell cycle – if excess cells exit proliferating pool too early, overall generation of neurons is reduced, and if too few exit during early rounds of division, then geometric expansion of proliferating population results in eventual overproduction of neurons o Lissencephaly – also called agyria because it is a smooth brain (no gyri or sulci) Can be caused by number of things One of best understood causes is mutations in gene encoding microtubule-associated protein LIS-1, which complexes with dynein and affects function of centrosome in nuclear movements Can occur from series of mutations in genes encoding enzymes responsible for glycosylation of α-dystroglycan – when receptor for extracellular matrix components does not have appropriate post-translational modifications, stability is diminished o Polymicrogyria – small, unusually numerous, irregularly formed cerebral convolutions Gray matter is composed of 4 layers (or fewer) with entrapment of apparent meningeal tissue at points of fusion that would otherwise be cortical surface Can be induced by localized tissue injury toward end of neuronal migration Can also be genetic (which would be bilateral and symmetric) o Neuronal heterotopias – group of migrational disorders commonly associated with epilepsy Consist of collections of neurons in inappropriate locations along migrational pathways One location heterotopias can be found is along ventricular surface, as though cells never managed to leave their place of birth Periventricular heterotopias – can be caused by mutations in gene encoding filamin A (actinbinding protein responsible for assembly of complex meshworks of filaments) Gene is on X chromosome – affected males die, and process of X inactivation separates neurons into those with normal allele (in correct location) and those with mutant allele (in heterotopia) o Mutations in doulbecortin (DCX – a microtubule-associated protein on the X chromosome) cause lissencephaly in males and subcortical band heterotopias in females Heterotopias may consist of discrete nodules of neurons sitting in subcortical white matter or complete ribbons that parody overlying cortex o Holoprosencephaly – incomplete separation of cerebral hemispheres across the midline Severe forms manifest midline facial abnormalities, including cyclopia Can be detected by ultrasound Can be caused by trisomy 13 or other genetic syndromes Mutations affecting SHH or its signaling pathway may also result in this o Arrhinencephaly – less severe version of holoprosencephaly – absence of olfactory cranial nerves and related structures o Agenesis of corpus callosum – relatively common – absence of white matter bundles that carry cortical projections from one hemisphere to the other Misshapen lateral ventricles (bat-wing deformity) Bundles of anteroposteriorly oriented white matter Can be associated with mental retardation, or patient may be completely normal Can be in isolation or associated with other malformations If they are born with this, they usually have minimal defects, but severing the corpus callosum during surgery later in life may cause problems Posterior Fossa Anomalies Dandy-Walker malformation – characterized by enlarged posterior fossa o Cerebellar vermis absent or present only in rudimentary form in anterior portion o Have large midline cyst that is lined by ependyma and is contiguous with leptomeninges on outer surface o Cyst represents expanded, roofless fourth ventricle in absence of normally formed vermis o Dysplasias of brainstem nuclei commonly found in association with this Arnold-Chiari malformation – also called Chiari type II malformation o Consists of small posterior fossa, misshapen midline cerebellum with downward extension of vermis through foramen magnum o Almost invariably has hydrocephalus and lumbar myelomeningocele o May include caudal displacement of medulla, malformation of tectum, aqueductal stenosis, cerebral heterotopias, and hydromyelia Chiari type I malformation – low-lying cerebellar tonsils extend down into vertebral canal o May be a silent abnormality or may cause symptoms referable to obstruction of CSF flow and medullary compression Syringomyelia and Hydromyelia Hydromyelia – characterized by discontinuous multi-segmental or confluent expansion of ependyma-lined central canal of cord Syringomyelia (syrinx) – formation of fluid-filled cleft-like cavity in inner portion of cord that may extend into brainstem (syringobulbia if it does) o May be associated with Chiari I malformation o May occur in association with intraspinal tumors or following traumatic injury Histological appearance is similar in both – destruction of adjacent gray and white matter, surrounded by dense feltwork of reactive gliosis Disease becomes manifest in second or third decade of life Distinctive signs and symptoms of syrinx – isolated loss of pain and temperature sensation in upper extremities because of predilection for early involvement of crossing anterior spinal commissural fibers of spinal cord Perinatal Brain Injury Brain injury occurring in perinatal period is important cause of childhood neurologic disability Injuries that occur early in gestation may destroy brain tissue without evoking usual reactive changes in parenchyma and may be difficult to distinguish from malformations Cerebral palsy – any nonprogressive neurologic motor deficit characterized by combinations of spasticity, dystonia, ataxia/athetosis, and paresis attributable to insults occurring during prenatal and perinatal periods o Signs and symptoms may not be apparent at birth and only be discovered as development proceeds o Can be caused by destructive lesions traced to remote events that may have caused hemorrhage and infarction Intraparenchymal hemorrhage – in germinal matrix, often near junction between thalamus and caudate nucleus o Premature infants have an increased risk o May remain localized or extend into ventricular system and thence to subarachnoid space, sometimes leading to hydrocephalus Infarcts may occur in supratentorial periventricular white matter (periventricular leukomalacia), especially in premature infants o Chalky yellow plaques consisting of discrete regions of white matter necrosis and calcification Multicystic encephalopathy – when both gray and white matter involved by extensive ischemic damage, and large destructive cystic lesions develop throughout hemispheres Perinatal ischemic lesions of cerebral cortex – depths of sulci bear brunt of injury and result in thinned-out, gliotic gyri (ulegyria) Basal ganglia and thalamus may suffer ischemic injury with patchy neuronal loss and reactive gliosis o Later, aberrant and irregular myelination gives rise to marble-like appearance of deep nueclei (status marmoratus) – because lesions are in caudate, putamen, and thalamus, choreoathetosis and related movement disorders are common Trauma-Induced Skull Fractures Displaced skull fracture – when bone is displaced into cranial cavity by distance greater than thickness of bone Relative incidence of fractures among skull bones is related to pattern of falls o When patient falls while awake (i.e., stepping off a ladder), the site of impact is often the occipital portion of the skull o A fall that follows loss of consciousness (i.e., fainting) commonly results in frontal impact Basal skull fracture typically follows impact to occiput or sides of head – can be indicated by lower CN malfunction, cervicomedullary region nerve damage, and presence of orbital or mastoid hematomas distant from point of impact CSF discharge from nose or ear and infection (meningitis) may follow skull fractures Diastatic – fractures that cross a suture Fracture lines of subsequent injuries do not extend across fracture lines of prior injury Parenchymal Injuries Concussion – altered consciousness secondary to head injury typically brought about by change in momentum of head (when moving head suddenly arrested by impact on a rigid surface) o Instantaneous onset of transient neurologic dysfunction, including loss of consciousness, temporary respiratory arrest, and loss of reflexes o Amnesia for event persists even after neurologic recovery is complete o Postconcussive neuropsychiatric syndromes can result with repetitive injuries Direct parenchymal injury – contusions and lacerations can be caused either through transmission of kinetic energy to brain and bruising analogous to what is seen in soft tissues or by penetration of an object and tearing of tissue o Blow to surface of brain transmitted through skull leads to rapid tissue displacement, disruption of vascular channels, and subsequent hemorrhage, tissue injury, and edema o Crests of gyri most susceptible because this is where direct force is greatest Most common locations for contusions correspond to most frequent sites of direct impact and regions of brain that overlie rough and irregular inner skull surface, such as frontal lobes along orbital ridges and temporal lobes Contusions less frequent over occipital lobes, brainstem and cerebellum unless as fracture contusions Coup injury – contusion at point of contact to a head blow Contrecoup injury – contusion on brain surface diametrically opposite to site of head blow If head is immobile during injury, only coup injury is found, but if head is mobile, both coup and contrecoup injuries may be found Sudden impacts result in violent posterior or lateral hyperextension of neck and may avulse pons from medulla or medulla from cervical cord, causing instantaneous death When seen in cross-section, contusions are wedge shaped, with broad base lying along surface, deep to point of impact o In earliest stages, there is edema and hemorrhage, often pericapillary o During next few hours, extravasation of blood extends throughout involved tissue, across width of cerebral cortex, and into white matter and subarachnoid space o Morphologic evidence of neuronal injury (pyknosis of nucleus, eosinophilia of cytoplasm, and disintegration of cell) takes about 24 hours to appear although functional deficits may occur earlier Axonal swellings develop in vicinity of damaged neurons or at great distances away Appearance of neutrophils followed by macrophages is as normal Plaque jaune – old traumatic lesions on surface of brain are depressed, retracted, yellowish brown patches involving crests of gyri most commonly located at sites of contrecoup lesions More extensive hemorrhagic regions of brain trauma give rise to larger cavitated lesions, which can resemble remote infarcts In sites of old contusions, gliosis and residual hemosiderin-laden macrophages predominate Diffuse axonal injury – injury to corpus callosum, paraventricular and hippocampal areas, cerebral peduncles, bracium conjunctivium, superior colliculi, and deep reticular formation of brainstem due to traumatic injury o Microscopic findings include axonal swelling and focal hemorrhagic lesions o Angular acceleration alone in absence of impact may cause diffuse axonal injury as well as hemorrhage o As many as 50% of people who develop coma shortly after trauma, even without cerebral contusions, are believed to have diffuse axonal injury o Mechanical forces associated with trauma damage integrity of axon at node of Ranvier with subsequent alterations in axoplasmic flow o Characterized by widespread but often asymmetric axonal swellings that appear within hours of injury and may persist for much longer Increased numbers of microglia in related areas of cerebral cortex and subsequent degeneration of involved fiber tracts Traumatic Vascular Injury Results from direct trauma and disruption of vessel wall, leading to hemorrhage Subarachnoid and intraparenchymal hemorrhages most often occur concomitantly in setting of brain trauma that results in superficial contusions and lacerations Traumatic tear of carotid artery where it traverses carotid sinus may lead to formation of arteriovenous fistula Dural arteries,especially the middle meningeal artery, are vulnerable to injury, particularly with temporal skull fractures in which fracture lines cross course of the vessel In children, temporary displacement of skull bones leading to laceration of a vessel can occur without skull fracture Once vessel is torn, extravasation of blood under arterial pressure can cause dura to separate from inner surface of skull o Expanding hematoma has smooth inner contour that compresses brain surface When blood accumulates over slowly, patients may be lucid for several hours before onset of neurologic signs Epidural hematoma may expand rapidly and is a neurological emergency requiring prompt drainage Bridging veins travel from convexities of cerebral hemispheres through subarachnoid space and subdural space to empty into superior sagittal sinus – these vessels are prone to tearing along their course through the subdural space and are source of bleeding in most cases of subdural hematoma o Venous sinuses are fixed, unlike skull which can float in CSF, and therefore displacement of brain in trauma can tear veins at point where they penetrate the dura o In elderly individuals with brain atrophy, bridging veins are stretched out and brain has additional space for movement, hence increased rate of subdural hematomas, even after relatively minor head trauma o Infants susceptible to subdural hematomas because bridging veins are thin-walled Acute subdural hematoma appears as collection of freshly clotted blood along brain surface, without extension into depths of sulci o Underlying brain is flattened and subarachnoid space often clear Venous bleeding is self-limited – breakdown and organization of hematoma take place over time o Lysis of clot (about 1 week) o Growth of fibroblasts from dural surface into hematoma (2 weeks) o Early development of hyalinized connective tissue (1-3 months) Typically, organized hematoma firmly attached by fibrous tissue only to inner surface of dura and not adherent to underlying smooth arachnoid, which does not contribute to its formation o Lesion can eventually retract as granulation tissue matures, until there is only thin layer of reactive connective tissue (subdural membranes) Common for subdural hematomas to have multiple episodes of repeat bleeding (chronic subdural hematomas) because of thin-walled vessels of granulation tissue – risk of repeat bleeding is greatest in first few months after initial hemorrhage Sequelae of Brain Trauma Post-traumatic hydrocephalus – largely due to obstruction of CSF resorption from hemorrhage into subarachnoid spaces Post-traumatic dementia and punch-drunk syndrome (dementia pugilistica) follow repeated head trauma during protracted period o Include hydrocephalus, thinning of corpus callosum, diffuse axonal injury, neurofibrillary tangles (mainly in medial temporal areas), and diffuse amyloid β-positive plaques Post-traumatic epilepsy, tumors (meningioma), infectious diseases, and psychiatric disorders may also occur Spinal Cord Trauma Most injuries of spinal cord associated with displacement of vertebral column, either rapid and temporary or persistent Segmental damage to descending and ascending white matter tracts isolates distal spinal cord from its cortical connections with cerebrum and brainstem – this is the principal cause of neurological deficits At level of injury, acute phase consists of hemorrhage, necrosis, and axonal swelling in surrounding white matter o Lesion tapers above and below level of injury o In time, central necrotic lesion becomes cystic and gliotic o Cord sections above and below lesion show secondary ascending and descending wallerian degeneration, respectively, involving long white-matter tracts affected at site of trauma