Investigating the Limits of Cell Growth Pre
advertisement

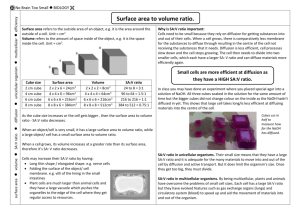
Investigating the Limits of Cell Growth Pre-lab Discussion In multicellular organisms, growth is accomplished by the production of more cells by cell division; mitosis followed by cytokinesis. Cell division will occur only when cells have reached a size large (Interphase G1 and G2) enough to ensure that the resulting daughter cells will have all of the necessary materials and structures for survival. The rate of exchange of nutrients (food, O2, H2O)entering the cell and waste (CO2, etc) leaving the cell through the cell membrane is determined by the cell’s surface area. And the rate at which these materials are used within the cell depends on the amount of amount of space within the cell, or the cell’s volume. Many factors, such as the amount of food and changes in temperature, can affect the cells growth. The purpose of this lab is to investigate how the surface area-to-volume ratio can limit cell growth. Materials Plastic spoon paper towel three agar-phenolphthalein cubes (1cm, 2cm, 3cm) Plastic knife sodium hydroxide solution 250mL beaker metric ruler Safety Precautions Put on safety goggles while working with sodium hydroxide solution and clean-up. Handle sodium hydroxide solution carefully, as it may irritate the skin or eyes. Rinse vigorously with water if you come in contact with the solution. Never taste anything in the lab. Procedure 1. Using a plastic knife, carefully pry the agar cube from the tray onto a paper towel. 2. BE CAREFUL NOT TO CRACK ANY OF YOUR CUBES!!! 3. Choose a lab partner who is detailed-oriented. You have one shot at the largest cube. Carefully, start with the largest cube and cut so that the sides are square to each other a cube that is a 3cm x 3cm x 3cm. 4. Then cut the medium sized cube 2cm x 2cm x 2cm and finally the smallest cube at 1cm x 1cm x 1cm. 5. Using your plastic spoon, carefully transfer the three cubes from the towel to the beaker. 6. Carefully pour the sodium hydroxide solution so that just enough solution covers the largest cube-DO NOT OVER FILL. (Caution: use extreme eye/skin care when handling any chemical. Wash with plenty of water when you come in contact with this solution and then tell the teacher.) 7. Wait for 7 minutes. 8. While the cubes are soaking use the following formulas to calculate surface area, volume, and surface area-tovolume ratio for each block. Show your work and answers in Data Table 1 Surface area (cm2) = number of cube sides x length x width Volume (cm3) = length x width x height Surface area-to-volume ratio (no units)= surface area/volume 9. After seven minutes, carefully pour the solution out into the sink with the water faucet flowing. Use the spoon or knife to prevent the cubes from falling out. BE CAREFUL NOT TO CRACK ANY OF YOUR CUBES!!! 10. Gently rinse the cubes twice with water. BE CAREFUL NOT TO CRACK ANY OF YOUR CUBES!!! 11. Finally, cut the cubes in half. Carefully measure the distance the pink color has diffused in each block and record in Data Table 2. Then measure the distance of the unpink square in the middle of the block. If your unpink section is a rectangle, measure the two sides and enter the average into Data Table 2. If soaked (pink) all the way through, enter zero cm. Data Table and Calculations Data Table 1 Surface Area (cm2) Cube Size Volume (cm3) Surface Area-to-Volume Ratio 3cm 2cm 1cm Data Table 2 Distance Pink Color Diffused into the Cube (cm) Cube Size Distance Unpink Center Section of Cube (cm) Volume of Unpink Center Cube (cm3) Percent Absorption of Solution 3cm 2cm 1cm Hint: To calculate the ”Volume of Unpink Center Cube” for each cube simply use the following formula: (“Distance of the Unpink Center Sectionof Cube”)3. This represents L x W x H To calculate the “Percent Absorption” for each cube use the following formula: [(Cube Size “Volume” minus “Volume of Unpink Center Cube)/Cube Size “Volume”] X 100 The Cube Size “Volume” data is from Data Table 1. The “Volume of Unpink Center Cube” data is from Data Table 2. Questions 1. Which cube has the greatest surface area? 2. Which cube has the greatest surface-to-volume ratio? 3. In which block did the pink color diffuse the most? Explain your answer. 4. What happened to the surface-to-volume ratio as the cube grew in size? 5. If the cubes were actual cells, which of the three would be most efficient in terms of allowing materials to enter and leave the cell. 6. Is it advantageous for a cell to be large or small. Explain your answer in terms of percent absorption. Extra Credit: Calculate the surface-to volume ratio for two cells that are 0.1cm and 0.01cm on a side. Which has the greater surface area in proportion to its volume? SHOW ALL WORK FOR FULL CREDIT.