Discrepant event -Fireworks in a Jar
advertisement

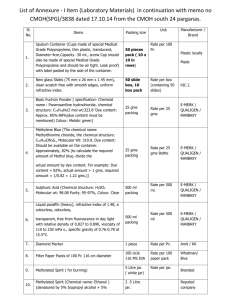
Fireworks in a Jar Fireworks in a Jar Fireworks in a jar is a great activity that will help lead to discussions about: 1. 2. 3. Density Mixtures Historical Events (Revolutionary War) This activity has been created to showcase and explore the concept of density in a fun and entertaining way. The student will get to see “fireworks” take place in a glass jar; and we didn’t even have to wait until the fourth of July. It is important that the students have been, or will be, introduced to the concept of density prior to this activity. Prompt students: “Can anyone tell me what density is; describe it to me in your own words. Which has a higher density, water or oil? How do you know? ATTENTION: Due to allergies, make sure that students do not come into direct contact with oil or oil products. If this activity is to be completed in small groups, make sure to seek parental consent for students to handle the needed materials. PS.2 The student will investigate and understand the nature of matter. Key concepts include a) the particle theory of matter; b) elements, compounds, mixtures, acids, bases, and salts; c) solids, liquids, and gases; d) physical properties; e) chemical properties; and f) characteristics of types of matter based on physical and chemical properties Materials: Vegetable oil, food coloring, pie tin pan, clear glass jar, plastic fork, water, access to technology (document camera, screen) Density: The mass per unit volume of a substance. It is often times affected by the temperature of the substance. *Oil is less dense than water. *Food coloring dissolves in water, but NOT in oil. *The glass jar should already be filled with warm water prior to starting the activity with students. Be cautious during handling and warn students that glass is fragile. Use the document camera to project activity as you model the first few steps. To begin, place tin pie pan on a flat surface. Pour in a half cup of oil. Prompt: “Who knows what happens when you add a drop of food coloring to water? Who knows what will happen if I were to add a drop of food coloring to the oil? Will it react the same way or differently? Tell me why you think that.” Add food coloring to oil (roughly eight to ten drops of food coloring) Prompt 2: “Why do you think that the food coloring is not mixing? Why? Will stirring it make a difference? Stir with a fork. “Did anything change?” Pour the mixture (oil and food coloring) into a glass jar. Make sure that students pay close attention to the jar. “Watch very closely, and see what happens next.” Fireworks!! The oil causes the food coloring to disperse at a slower rate; it looks like a sudden burst. And because oil is less dense than water and oil floats, the fireworks happen near the top and middle of the jar of water.