ijag12037-sup-0001-SupportingInfo

Supplemental Information for Lithium Thiophosphate Glasses and Glass-Ceramics as
Solid Electrolytes: Processing, Microstructure, and Properties
Seth S. Berbano
†
, Mehdi Mirsaneh, Michael T. Lanagan, Clive A. Randall
Department of Materials Science & Engineering, Center for Dielectric Studies,
Materials Research Institute
The Pennsylvania State University, Millennium Science Complex
University Park, PA 16802
Phone: 814-863-1328, Fax: 814-865-8126
Footnotes: This material is based on work supported by the National Science Foundation (NSF) as part of the Center for Dielectric Studies (Grant No. 0628817), NSF Graduate Research
Fellowship (Grant No. DGE-1255832), NSF ASSIST (Grant No. EEC-1160483), 3M Science and Technology Fellowship, and Department of Energy Graduate Automotive Technology
Education Fellowship (Grant No. DE-EE0005575 ) . Any opinion, findings, and conclusions or recommendations expressed are those of the authors and do not necessarily reflect the views of the NSF.
Authors thank Prof. Angela Lueking (planetary mill), Prof. Ramakrishnan Rajagopalan
(glovebox), and technical sta ff at Penn State’s Materials Research Institute, including Julie
Anderson (particle size analysis), Amanda Baker (lab assistance), Jeff Long (impedance spectroscopy), Steve Perini (heated die), and Nichole Wonderling (XRD). Thanks to the NSF
International Materials Institute for New Functionality in Glass (NSF DMR 0844014) for the
International Conference Travel Scholarship to present this work in July 2013 at the
23 rd
International Congress on Glass in Prague, Czech Republic.
† Author to whom correspondence should be directed: sethberb@psu.edu
1
SI. Experimental methods
(1) Synthesis by mechanical milling
Mechanically milled products were prepared by weighing appropriate amounts of Li
2
S
(Alfa Aesar, 99.9% metals basis, ~ 74 μm) and P
2
S
5
(Sigma Aldrich, 99%) inside an Ar-filled glovebox. The powders were ground for five minutes in an agate mortar and pestle and then loaded into an 80 mL ZrO
2
pot. Twenty YSZ balls, 10 mm in diameter, were used as the milling media. The sample to milling media weight ratio was ~ 1:13. The pot was sealed under vacuum with a rubber gasket and taped shut to avoid air influx and contamination while the sample was milled outside the glovebox. Samples were mechanically milled at 370 rpm for 14, 20, or 26 hr. using a high-energy planetary mono-mill (Pulverisette 6, Fritsch, Idar-Oberstein, Germany).
(2) X-ray diffraction
A quartz low-background support covered with Kapton (Chemplex Industries, Palm City,
FL) was characterized without any sample.
Inside the glovebox, powder samples were loaded in the cavity of a quartz low-background support and covered with Kapton. Room temperature Xray diffraction (XRD) patterns were collected using a
θ
/
θ
goniometer with Cu-K
α
radiation, fixed slit incidence (0.5 deg. Divergence, 1.0 deg. Anti-scatter, specimen length 10 mm) and diffracted (0.5 deg. Anti-scatter, 0.02 mm nickel filter optics), (X’Pert Pro MPD, PANalytical,
Westborough, MA ). Data was collected at 45 kV and 40 mA from 25-80 deg. 2
θ
using a PIXcel detector in scanning mode with a PSD length of 3.35 deg. 2
θ
and 255 active channels for a duration of ~ 15 minutes.
2
(3) Pressure-forming
Particle size analysis was performed on mechanically milled powder. (Malvern
Mastersizer S, Malvern Instruments Ltd., Malvern, UK). Mechanically milled powder was loaded into a hardened steel die with a light PTFE spray coating (Dry Film Release Agent,
Sprayon) and a thermocouple that screwed into the die’s inner sleeve (EQ-Die12-HC, MTI,
Richmond, CA). A 93 MPa pre-press was performed on Tygon supports, causing a friction fit between the upper and lower punch. Next, the heating jacket was attached to the double-action die. When pressurized to 186 MPa, the die was heated at ~ 12 o
C/min from room temperature to the desired temperature. Pressure intermittently decreased primarily from the softening of the glassy powder and minimally from the change in the die-to-punch fit from differential thermal expansion. Pressure was increased back to 186 MPa during heating. After reaching the desired temperature, the heater was turned off and the die was allowed to cool to room temperature.
Pressure-formed pellets were polished ~ 200 μm below the surface to remove compositional inhomogeneities, such as lithium depletion, caused by pressure-forming. Last, geometric density was determined by massing 1/2 in. diameter pellets on a balance and measuring their thickness using calipers. All pressure-forming took place inside the glovebox.
(4) Impedance spectroscopy
Pellet faces (1/2 in. diameter) were electroded with air-dry Ag paste (Premetek, State
College, PA) and allowed to dry overnight in the glovebox antechamber. Pellets (1.5 to 2.5 mm thick) were loaded in a custom-built sample cell assembly consisting of brass covers, brass spacers, a Teflon guard, a Teflon spacer, silicone o-rings, and a compression spring. The sample cell was transferred from the glovebox to a N
2
-purged, temperature-controlled chamber (9023,
Delta Design, Poway, CA). Thermal equilibrium of the sample was determined from overlapping
3
impedance spectra. Complex impedance spectra were measured using an impedance analyzer (SI
1287 and SI 1255B FRA, Solatron Instruments, Oak Ridge, TN) with a sinusoidal voltage amplitude 100 mV vs. OCV for -120 o
C < T < -10 o
C and 1 Hz < f < 1 MHz. Data were corrected for sample geometry and analyzed using ZView (Version 2.2, Scribner Associates Inc., Southern
Pines, NC).
4
SII. Figure list
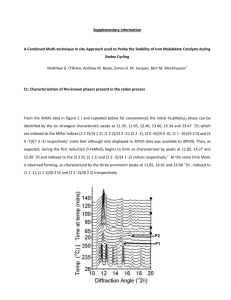
Figure S1.
X-ray diffraction powder patterns of Li
2
S and P
2
S
5
starting materials, mechanically milled Li
2
S (to show lack of texture), mechanically milled 0.70 Li
2
S + 0.30 P
2
S
5
, and ground powder from a 0.70 Li
2
S + 0.30 P
2
S
5
pellet that was pressure formed above the glass transition temperature (216 o
C).
Figure S2.
Geometric density (◊) and percent theoretical density (∆) of Li
7
P
3
S
11
value from
Yamane et al. S1 rather than the value from Lepley and Holzwarth S2 vs. maximum die temperature. The glass transition temperature (216 o
C), obtained from Differential Scanning
Calorimetry thermogram, is indicated in the inset.
Figure S3. Overlay of impedance complex plane plot for 72% (◊) and 94% (∆) dense pellets (of
Li
7
P
3
S
11
value from Yamane et al.
S1
) at –50 o
C. The frequencies corresponding to tan δ max
and
M” max
are indicated.
Figure S4.
Geometry-normalized imaginary impedance and electrical modulus vs. logarithm of frequency showing thermal activation of Z
″
(◊) and M
″
(∆) relaxation peak values for 72% (left) and 94% dense (right) pellets of Li
7
P
3
S
11
value from Yamane et al.
S1
. The Z
″
scale changes an order of magnitude between each temperature frame, while the M
″
scale remains unchanged.
Instrument ranging offsets at ~ 30 kHz, which do not affect the frequencies of M ″ peaks, have been smoothed.
5
Figure S1. X-ray diffraction powder patterns of Li
2
S and P
2
S
5
starting materials, mechanically milled Li
2
S (to show lack of texture), mechanically milled 0.70 Li
2
S + 0.30 P
2
S
5
, and ground powder from a 0.70 Li
2
S + 0.30 P
2
S
5
pellet that was pressure formed above the glass transition temperature (216 o
C).
6
1.2
1.0
0.8
0.6
0.4
0.2
0.0
-0.2
-0.4
-0.6
-0.8
-1.0
-1.2
-1.4
100
0.70Li
2
S + 0.30P
2
S
5
Milled 14 hr
125 150
T g
~216 o
C t = 20 o
C/min
175 200 225
Temperature ( o
C)
250
T c
~257 o
C
Li
7
P
3
S
11
275 300
Figure S2.
Geometric density (◊) and percent theoretical density (∆) of Li
7
P
3
S
11
value from
Yamane et al.
S1
rather than the value from Lepley and Holzwarth
S2 vs. maximum die temperature. The glass transition temperature (216 o C), obtained from Differential Scanning
Calorimetry thermogram, is indicated in the inset.
7
Figure S3.
Overlay of impedance complex plane plot for 72% (◊) and 94% (∆) dense pellets (of
Li
7
P
3
S
11
value from Yamane et al.
S1
) at –50 o
C. The frequencies corresponding to tan δ max
and
M” max
are indicated.
8
5.0x10
7
4.0x10
7
3.0x10
7
2.0x10
1.0x10
7
7
-70oC
72% Dense
1.0x10
8.0x10
6.0x10
11
10
10
4.0x10
10
2.0x10
10
0.0
5.0x10
4.0x10
3.0x10
2.0x10
1.0x10
6
6
6
6
6
-Z" (
cm )
M"/
o
( F-1
cm)
0.0
1.0x10
11
8.0x10
10
6.0x10
10
4.0x10
10
2.0x10
10
-50oC
0.0
5.0x10
5
0.0
1.0x10
11
4.0x10
5
8.0x10
10
3.0x10
5
6.0x10
10
2.0x10
5
4.0x10
10
1.0x10
5
2.0x10
10
0.0
5.0x10
4
-30oC
0.0
1.0x10
11
4.0x10
4
8.0x10
10
3.0x10
4
6.0x10
10
2.0x10
4
1.0x10
4
0.0
10
-1
-10oC
10
0
10
1
10
2
10
3
Frequency (Hz)
10
4
4.0x10
2.0x10
10
5
0.0
10
6
10
10
5.0x10
8
4.0x10
8
3.0x10
8
2.0x10
8
1.0x10
8
0.0
5.0x10
7
-110oC
94% Dense
2.0x10
1.5x10
1.0x10
5.0x10
11
11
11
10
4.0x10
7
3.0x10
7
2.0x10
7
1.0x10
7
0.0
5.0x10
6
-90oC
-Z" (
cm)
M"/
o
(F-1-cm)
0.0
2.0x10
1.5x10
1.0x10
5.0x10
0.0
2.0x10
11
11
11
10
11
4.0x10
6
3.0x10
6
1.5x10
11
1.0x10
11
2.0x10
6
1.0x10
6
0.0
5.0x10
5
-70oC
5.0x10
10
0.0
2.0x10
11
4.0x10
5
1.5x10
11
3.0x10
5
1.0x10
11
2.0x10
5
1.0x10
5
0.0
10
-1
-50oC
10
0
10
1
10
2
10
3
10
Frequency (Hz)
4
5.0x10
10
5
0.0
10
6
10
Figure S4.
Geometry-normalized imaginary impedance and electrical modulus vs. logarithm of frequency showing thermal activation of Z
″
(◊) and M
″
(∆) relaxation peak values for 72% (left) and 94% dense (right) pellets of Li
7
P
3
S
11
value from Yamane et al. S1 . The Z ″ scale changes an order of magnitude between each temperature frame, while the M
″
scale remains unchanged.
Instrument ranging offsets at ~ 30 kHz, which do not affect the frequencies of M ″ peaks, have been smoothed.
9
Table S1. Ionic conductivity properties of 0.70 Li
2
S + 0.30 P
2
S
5
synthesized by various methods
Reference
σ
25 o
C
(Ω-cm) -1
σ o
(Ω-cm) -1
ΔE
Act
(eV)
Comments
†
Mizuno et al.
S3 5.4 x 10 -5
Zhang and Kennedy S4 1.60 x 10 -4
Trevey et al.
S5
Minami et al.
Seino et al.
S7
Mizuno et al.
S6
S3
Minami et al.
S6
Minami et al.
S8
2.5 x 10 -4
4.1 x 10 -4
1.0 x 10 -3
3.2 x 10 -3
4.1 x 10 -3
5.2 x 10 -3
10 4.2*
10 5.34
- - -
10 3.2*
10 2.1‡
10 0.66*
10
10
‡
0.067*
0.39
0.37
- - -
0.33
0.30
‡
0.19
0.15
0.12
‡
MM glass
MQ glass
SSBM 55 o C 20 hr., partially crystalline
Crystallized from melt 700 o C 48 hr. then quenched
MQ glass, crystallized 290 o C 5 hr.
MM glass, crystallized 240 o C 2 hr.
MQ glass, crystallized 360 o C 1 hr.
MM, 210 o C 0.5 hr. then 280 o C 1 hr. in a hot press
Table S1 footnotes : Data is presented as 𝜎 = 𝜎 𝑜 𝑒𝑥𝑝 (
−𝛥𝐸
𝐴𝑐𝑡 𝑘
𝐵
𝑇
) rather than 𝜎 = [𝜎 𝑜
𝑇]𝑒𝑥𝑝 (
−𝛥𝐸
𝐴𝑐𝑡 ) and ordered by σ 𝑘
𝐵
𝑇
25 o
C
. In literature, only Yamane et al. S1 has reported an experimental relative density value of 96% of Li
7
P
3
S
11
in a crystallized sample. Their data is not included in the table since no direct conductivity measurements were reported in their paper S1 .
†
MM = Mechanically milled, MQ = Melt-quenched, SSBM = Single-step ball milled
* Calculated from σ
25 o
C
and ΔE
Act
, assuming Arrhenius temperature dependence.
‡ Estimated from an Arrhenius plot.
10
SIII. References
S1 H. Yamane , M. Shibata, Y. Shimane, T. Junke, Y. Seino, S. Adams, K. Minami, A. Hayashi, and M. Tatsumisago, “Crystal Structure of a Superionic Conductor, Li
7
P
3
S
11
,”
Solid State Ionics ,
178 , 1163-7 (2007).
S2 N. D. Lepley and N. A. W. Holzwarth, “Computer Modeling of Lithium Phosphate and
Thiophosphate Electrolyte Materials,”
J. Electrochem. Soc .
, 159, A538-47 (2012).
S3 F. Mizuno, A. Hayashi, K. Tadanaga, and M. Tatsumisago, “New, Highly Ion-Conductive
Crystals Precipitated from Li
2
S–P
2
S
5
Glasses,” Adv. Mater.
, 17 , 918-921 (2005).
S4 Z. Zhang and J.H. Kennedy, “Synthesis and Characterization of the B
2
S
3
-Li
2
S, the P
2
S
5
-Li
2
S and the B
2
S
3
-P
2
S
5
-Li
2
S Glass Systems,”
Solid State Ionics , 38 , 217-224 (1990).
S5
J. Trevey, J.S. Jang, Y.S. Jung, C.R. Stoldt, and S.H. Lee, “Glass–Ceramic Li
2
S–P
2
S
5
Electrolytes Prepared by a Single Step Ball Billing Process and their Application for All-Solid-
State Lithium–Ion Batteries,” Electrochem. Comm.
, 11 , 1830-1833 (2009).
S6
K. Minami, A. Hayashi, and M. Tatsumisago, “Preparation and Characterization of Superionic
Conducting Li
7
P
3
S
11
Crystal from Glassy Liquids,” J. Ceram. Soc. Jpn.
, 118 , 305-308 (2010).
S7
Y. Seino, K. Takada, B.C. Kim , L. Zhang, N. Ohta, H. Wada, M. Osada, and T. Sasaki,
“Synthesis of Phosphorous Sulfide Solid Electrolyte and All-Solid-State Lithium Batteries with
Graphite Electrode, “
Solid State Ionics , 176 , 2389-2393 (2005).
S8 K. Minami, A. Hayashi, and M. Tatsumisago, “Crystallization Process for Superionic Li
7
P
3
S
11
Glass-Ceramic Electrolytes,” J. Am. Ceram. Soc., 94 , 1779–1783 (2011).
11
SIV. Additional comments on figures in the manuscript “Lithium Thiophosphate Glasses and Glass-Ceramics as Solid Electrolytes: Processing, Microstructure, and Properties”
Figs. 1 and 2 contain original data by Berbano et al. of the densification behavior with references denoting the relative densities and obtained phases (verified with x-ray diffraction in
Fig. 8).
Fig. 3 is a known crystal structure. A very low lithium-ion vacancy migration energy of
0.15 eV has been calculated in the b -direction 24 . One research challenge is to find a way to texture Li
7
P
3
S
11
in the b -direction.
Figs. 4, 5, 6, and 7 have been reproduced from literature with minor modifications.
Appropriate licenses were required for their reprinting. Fig. 4 was published in the Journal of the
American Ceramic Society and does not require a reprint license for the International Journal of
Applied Glass Science . Their inclusion enhances the discussion of the current state of knowledge about lithium thiophosphate microstructure and compositional trends of ionic conductivity (and whether bulk or surface conduction is important).
Fig. 8 contains original data by Berbano et al., which is the first to show powder diffraction of crushed pellets after pressure-forming. References were used for phase identification.
Fig. 9 contains original data by Berbano et al.
Fig. 10 contains original data by Berbano et al. and appropriate references for the relative density values.
12