My thoughts re rehab programme pilot
advertisement

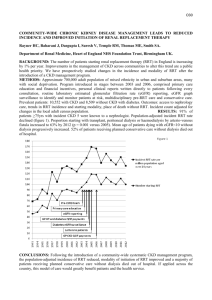
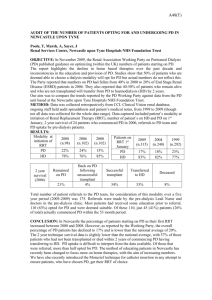
P94 JOINT ANALYSIS OF LONGITUDINAL eGFR MEASUREMENTS AND TIME TO RENAL REPLACEMENT THERAPY AND DEATH IN A CKD COHORT Ozgur Asar1, James Ritchie2, Philip Kalra2, Peter J. Diggle1,3 1 Lancaster Medical School, Lancaster University, UK 2 Vascular Research Group, Manchester Academic Health Sciences Centre, University of Manchester, Salford Royal NHS Foundation Trust, UK 3 Institute of Infection and Global Health, University of Liverpool, UK INTRODUCTION: Longitudinal eGFR measurements and time to renal replacement therapy (RRT) and/or death are typically analysed separately. Moreover, eGFR measurements are subject to substantial measurement error and are typically measured at irregular time points. The so-called joint models for longitudinal and survival data enable investigation of the relationship between eGFR trajectories and the hazard for RRT and/or death whilst making allowance for the inherent imprecision of eGFR measurements. Prognostic features of eGFR trajectories such as its rate of change over time can be easily accommodated within the survival part of a joint model. In this study, we aim to introduce joint models to the nephrology community. METHODS: We compare three approaches: 1) standard Cox proportional hazards model with baseline eGFR as an explanatory variable, 2) time-varying survival models using current eGFR as an explanatory variable, and 3) a joint model, in which a linear mixed effects model for longitudinal eGFR is combined with a Cox proportional hazards model to enable unbiased estimation of the relationship between aspects of a patient’s underlying error-corrected GFR trajectory and their hazard for survival. We apply the methods to data from the Chronic Renal Insufficiency Standards Implementation Study (CRISIS), a prospective study of outcome in a referred CKD population. Baseline explanatory variables include sex, age, smoking status, alcohol consumption and co-morbidities. Survival outcomes considered are RRT, death and the composite end-point of RRT or death. RESULTS: Baseline data for 1611 patients from CRISIS were considered, with a total of 3154 follow-up points. Median age at recruitment was 67.2 years [IQR 55.6-74.9], with a mean baseline eGFR of 32.7±15.1 ml/min. Over a median follow up period of 3.9 years [IQR 2.2-5.6], 19% of patients progressed to RRT, and 33% of patients suffered death before RRT. The findings with respect to the association between renal function and hazard for initiation of RRT are summarised in Table 1. The standard Cox model and the time-varying survival model performed similarly; in each case, the measurement errors in baseline and current eGFR led to shrinkage of the associated hazard ratios towards 1. In the joint modelling, inclusion of rate of change in GFR in addition to current value of GFR (Joint 2) gave a significant improvement in model fit compared with a model that included only current GFR (Joint1, likelihood ratio test, p =0.01). Table 1. Results for the survival processes in terms of eGFR for RRT. Presented results are hazard ratios and related 95% confidence intervals. eGFR Rate of change Max log-lik* Standard Cox Time-varying survival Joint1 Joint2 0.882 (0.870, 0.894) 0.837 (0.823, 0.852) 0.759 (0.729, 0.790) 0.777 (0.749, 0.806) 0.785 (0.684, 0.900) -12,921.58 -12,918.02 * Maximized log-likelihood. Greater values indicate better model fit; for formal test, see likelihood ratio test in the text. CONCLUSION: Joint models enable optimal use of repeated measurement and time to event data. They are particularly useful when repeated measurements are made at irregular follow-up times and are measured with appreciable error, both of which are typical features of observational studies in renal medicine.