Chapter 11 gases
advertisement

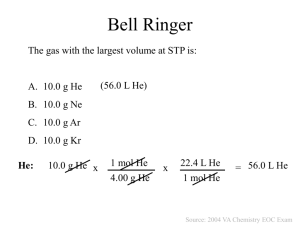
Chapter 11: Physical Properties of Gases Objectives Describe how the kinetic-molecular theory of matter explains ideal gases Differentiate between ideal and real gases Describe temperature & pressure and convert units. State the conditions of STP Explain the relationship between volume, temperature and pressure of gases Calculate the relationship of V, T, P using Boyle’s, Charles’, and Gay Lussac’s laws Perform the calculations with the combined gas law Calculate partial pressures from Dalton’s law. A. Kinetic-Molecular Theory of Ideal Gases Particles in an ideal gas … are ______________________ relative to their size. have elastic collisions (_______________________________________________). are in constant, random, rapid ___________________________. don’t ___________________________ each other. have an avg. KE directly related to ____________________________. B. Real Gases Particles in a REAL gas… have their own ____________________________ _________________________ each other Gas behavior is most ideal… at low ______________________ at high _____________________ in nonpolar _________________________ (He, Ne, H 2, N2, I2 etc.) C. Characteristics of gases • Gases _____________________ any container. – random motion, no attraction • Gases are ______________________ (like liquids). – no attraction • Gases have very low ____________________________. – no volume = lots of empty space D. Temperature • Gases can be __________________________. – no volume = lots of empty space • Gases undergo ____________________________________. - Random motion Always use absolute temperature E. Pressure ___________________________ - Measures atmospheric pressure ____________________________ - Invented by Key units at sea level F. STP G. Dalton’s Law of Partial Pressures STP means: A gas is collected over water at a temp of 35.0°C when the barometric pressure is 742.0 torr. What is the partial pressure of the dry gas? Law: Given: Work: H. Boyle’s Law The pressure and volume of a gas are ___________________ related - at constant mass & temp I. Charles’ Law The volume and absolute temperature (K) of a gas are ________________ related - at constant mass & pressure J. Gay Lussac’s Law The pressure and absolute temperature (K) of a gas are _________________ related - at constant mass & volume CONCEPT CHECK Pressure decreases… Temperature increases… Volume increases… Pressure decreases… Temperature decreases… Volume increases… K. Combined Gas Law SAMPLE PROBLEMS: A gas occupies 473 cm3 at 36°C. Find its volume at 94°C. Law: Given: Work: 3. A gas occupies 100. mL at 150. kPa. Find its volume at 200. kPa. Law: Given: Work: A gas occupies 7.84 cm3 at 71.8 kPa & 25°C. Find its volume at STP. Law: Given: L. Avogadro’s Law Work: Equal Volumes of gases contain ________________________. - At ___________________________ M. The Ideal Gas Law R = Universal Gas Constant R=0.0821 Latm/molK R=8.315 LkPa/molK n = number of moles Ideal Gas Law Sample Problems 1. Calculate the pressure in atmospheres of 0.412 mol of He at 16°C & occupying 3.25L. Given: Work: 2. Find the volume of 85 g of O2 at 25°C and 104.5 kPa. Given: Work: