Divergence and Natural Selection of Autocatalytic Primordial
advertisement

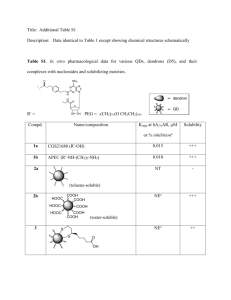
Electronic supplementary material Divergence and Natural Selection of Autocatalytic Primordial Metabolic Systems Sergey A. Marakushev · Ol’ga V. Belonogova Institute of Problem of Chemical Physics, Russian Academy of Sciences, 142432 Chernogolovka, Moscow Region, Russia The major equilibrium factors in parageneses physicochemical analysis (Marakushev and Belonogova 2009) are temperature, pressure, and the chemical potentials (µi) of components that represent its partial energy. The value µi is expressed through activity I, and fugacity fi as follows: µi =(µ0i)Т,р + RTlni = (µ0i) Т, р + RTln fi. Here, numerical values depend on conventional standard states. For activity, the state of unit molal concentration is usually considered the standard state at a given temperature and pressure. In this state i = 1, and hence, µi = (µ0i)Т,р. The standard state accepted in the present study for aqueous substances corresponds to unit activity of a substances in a hypothetical one-molal solution referenced to infinite dilution at any temperature and pressure. The activity of liquid H2O was taken to be 1. Table Values of Gibbs free energies of formation ΔG0T (kJ/mol) of aqueous substances at elevated temperatures and saturated vapor pressure (PSAT) Т, К Aqueous substances 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 CH3COO– CH3COOH CH2(COO–)2 CH2(COOH)2 (CH2)2(COO–)2 (CH2)2(COOH)2 (CH2)3(COO–)2 (CH2)3(COOH)2 CHOCOO– CHOCOOH СH2CH(OH)(COO–)2 СH2CH(OH)(COOH)2 (CH2)2CO(COO–)2 (CH2)2CO(COOH)2 CH2CO(COO–)2 CH2CO(COOH)2 (CH)2(COO–)2 (CH)2(COOH)2 CH3COCOO– CH3COCOOH 298 -369.32 -396.10 -685.18 -733.97 -687.77 -743.92 -683.97 -739.66 -460.30 -467.54 -846.14 -902.29 -801.26 -857.06 -798.40 -854.54 -605.24 -659.42 -474.44 -481.68 373 -376.10 -411.02 -686.83 -752.86 -692.07 -765.41 -690.76 -764.92 -467.12 -478.25 -852.33 -925.67 -810.97 -884.26 -808.65 -881.99 -604.85 -678.19 -485.69 -496.82 423 -380.79 -422.51 -685.36 -766.95 -692.89 -781.64 -693.64 -783.99 -477.08 -492.23 -853.05 -941.80 -808.38 -896.10 -800.45 -889.23 -605.52 -694.27 -497.16 -509.75 473 -385.39 -435.10 -681.53 -782.04 -691.75 -799.16 -694.82 -804.54 -484.74 -505.21 -851.62 -959.03 -803.65 -912.48 -795.38 -904.59 -604.21 -711.62 -503.94 -524.40 To date, standard molal thermodynamic properties at elevated temperatures and pressures were studied for hundreds of aqueous substances. Internal consistent values of free energy of formation are now available for aqueous organic substances of the C−H−O system, including hydrocarbons, carboxylic acids, ketones, alcohols, and aldehydes. The standard partial molal Gibbs free energies of formation (ΔG0T) at elevated temperatures and PSAT for substances (1-8), aldehydes, and hydrocarbons are from Amend and Shock (2001), Schulte and Shock (1993), and Oelkers et al. (1995). The free energy values (ΔG0298) of substances formation (9-20) in their ionized forms under standard conditions (298 K and 1 bar) are from Miller and Smith-Magowan (1990). ΔG0T values of substances formation (9-20) are calculated by the group additivity algorithm that uses ΔG0T values of groups formation (Amend and Helgeson 1997a,b). For example, the nonionized fumarate formula (CH)2(COOH)2 can be conventionally represented as an ethylene formula by replacing two terminal hydrogen atoms for carboxylic groups (-COOH). Thus, the free energy of fumarate formation in the nonionized form can be calculated using the following formula: ΔG0(CH)2(COOH)2 = ΔG0C2H4 + 2ΔG0(-COOH) – 2ΔG0(-H), where ΔG0(-H) = ΔG0(CH3) – ΔG0(-CH2-). To obtain a ΔG0 value of fumarate in the ionized form, we use the difference (ΔG0(CH2)2(COOH)2 – ΔG0(CH2)2(COO–)2). Similarly, we can consider the following substances (9-20) and use the appropriate dicarboxylic acids as the basis for our calculations. Thus, a trend of (ΔG0T) temperature curve is determined by the temperature dependence ΔG0T of the acids in their ionized and nonionized form (Amend and Shock 2001). References Amend JP, Helgeson HC (1997a) Group additivity equations of state for calculating the standard molar thermodynamic properties of aqueous organic species at elevated temperatures and pressures. Geochim et Cosmochim Acta 61:11 - 46 Amend JP, Helgeson HC (1997b) Calculation of the standard molal thermodynamic properties of aqueous biomolecules at elevated temperatures and pressures. Part 1 L-a-Amino acids. J Chem Soc Faraday Trans 93: 1927 – 1941 Amend JP, Shock EL (2001) Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol Rev 25:175-243 Marakushev SA, Belonogova OV (2009) The parageneses thermodynamic analysis of chemoautotrophic СО2 fixation archaic cycle components, their stability and self-organization in hydrothermal systems. J Theoret Biol 257: 588–597 Miller SL, Smith-Magowan D (1990) The Thermodynamics of the Krebs Cycle and Related Compounds. J Phys Chem Ref Data 19: 1049-1073 Oelkers EH, Helgeson HC, Shock EL et.al. (1995) Summury of the apparent standard partial molal Gibbs free energies of the formation of aqueous species, minerals, and gases at pressures 1 to 5000 bars and temperatures 25 to 1000 oC. J Phys Chem Ref Data 24: 1401–1560 Schulte MD, Shock EL (1993) Aldehydes in hydrothermal solution: Standard partial molal thermodynamic properties and relative stabilities at high temperatures and pressures. Geochim et Cosmochim Acta 57: 3835-3846