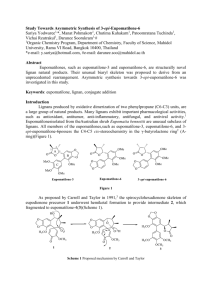
Study Towards Asymmetric Synthesis of 3-epi-Eupomatilone
advertisement

Study Towards Asymmetric Synthesis of 3-epi-Eupomatilone-6 Sariya Yodwaree1,*, Manat Pohmakotr1, Chutima Kuhakarn1, Patoomratana Tuchinda1, Vichai Reutrakul1, Darunee Soorukram1,# 1 Organic Chemistry Program, Department of Chemistry, Faculty of Science, Mahidol University, Rama VI Road, Bangkok 10400, Thailand *e-mail: y.sariya@hotmail.com, #e-mail: darunee.soo@mahidol.ac.th Abstract Eupomatilones, such as eupomatilone-3 and eupomatilone-6, are structurally novel lignan natural products. Their unusual biaryl skeleton was proposed to derive from an unprecedented rearrangement. Asymmetric synthesis towards 3-epi-eupomatilone-6 was investigated in this study. Acknowledgements: Financial support from the Thailand Research Fund (to D.S., MRG5580046), the Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative, Mahidol University, and the Center of Excellence for Innovation in Chemistry (PERCH-CIC) are gratefully acknowledged. Keywords: eupomatilone, lignan, conjugate addition References 1. 2. 3. Hong S, McIntosh C. An approach to the synthesis of theeupomatilones. Org. Lett. 2002; 4:19-21. Johnson B J, BercotA E, Williams M C,Rovis T. A concise synthesis of eupomatilones 4, 6, and 7 by rhodium-catalyzed enantioselectivedesymmetrization of cyclic mesoanhydrides with organozinc reagents generated in situ.Angew. Chem. Int. Ed. 2007; 46:4514-4518. Coleman R S, Gurrala S R. Total synthesis of eupomatilones 4 and 6: structurally rearranged and atroppisomerically fluxional lignan natural products. Org. Lett. 2004; 6:4025-4028.