6. Mass Transfer 6.1 Introduction Figure (1) Nature tends to equalize
advertisement

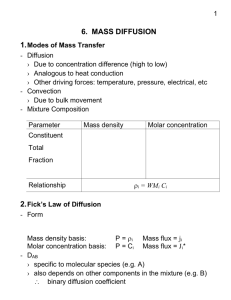
6. Mass Transfer 6.1 Introduction It is important to distinct between mass transfer and the bulk fluid motion (or fluid flow). Fluid flow occurs on a macroscopic level as a fluid is transported from one location to another. Mass transfer requires the presence of two regions at different chemical composition, and mass transfer refers to the movement of a chemical species from a high concentration region Figure (1) Nature tends to equalize things by toward a lower concentration one relative to other forcing a flow from the high to the low chemical species present in the medium. The primary concentration driving force for fluid flow is the pressure difference, whereas for mass transfer it is the concentration difference. Therefore, there is no mass transfer in homogenous medium. As shown in figure (1), the species flow is always in the direction of decreasing concentration; that is from the region of high concentration to the region of low concentration. The rate of flow of a species is proportional to the concentration gradient dC/dx, and the area A normal to flow direction and is expressed as: Flow rate ∝ (Normal area)×(Concentration gradient) or 𝑑𝐶𝐴 𝑚̇𝑑𝑖𝑓𝑓 = −𝐷𝐴𝐵 𝐴 𝑑𝑥 Where DAB is the proportional constant and is called the diffusion constant. The foregoing equation is similar to Fourier’s law of conduction. This equation, tell us how much the rate of mass of component A, at which species A diffused in the mixture of A and B, of course in the direction of lower value concentration of A in this mixture. Mass Transfer can occur in liquids and solids as well as in gas. Typically diffusion rates in gases much higher than they are in liquids and much higher in liquids than in solid. Accordingly, diffusion coefficients in gas mixtures are a few orders of magnitude larger Figure (2) Some examples of mass transferthat involve a liquid and /or a solid than these of liquid or solid solution, see figure (2). 6.2 Analogy between Heat and Mass Transfer The mechanisms of heat and mass transfer are analogous to each other and thus the heat transfer knowledge may be helpful to solve mass transfer problems. In the following sections, the similarity between heat and mass transfer is explained. Temperature The driving force for heat transfer is the temperature difference. In contrast, the driving force for mass 1 Figure (3) Analogy between heat and mass transfer transfer is the concentration difference. According to this analogy, the temperature is a measure of heat concentration, see figure (3). Conduction As it is known, heat is transferred by conduction, convection and radiation. Mass, however, is transferred by conduction (called diffusion) and convection only and there is no radiation mass transfer. The rate of heat conduction in a direction x is proportional to the temperature dradient dT/dx in that direction and is expressed by Fourier’s law of conduction as: 𝑄̇𝑐𝑜𝑛𝑑 = −𝑘 𝐴 𝑑𝑇 𝑑𝑥 Where k is the thermal conductivity of the medium and A is the area normal to the direction of heat transfer. (4) Analogy between heat Likewise, the rate of mass diffusion 𝑚̇𝑑𝑖𝑓𝑓 of a chemical Figure conduction and mass diffusion species A in a stationary medium in direction x is proportional to the concentration gradient dc/dx in that direction and is expressed by Fick’s law of diffusion as: (see figure (4)) 𝑚̇𝑑𝑖𝑓𝑓 = −𝐷𝐴𝐵 𝐴 𝑑𝐶𝐴 𝑑𝑥 Where 𝐷𝐴𝐵 is the diffusion coefficient (or mass diffusivity) of species in mixture and 𝐶𝐴 is the concentration of the species in the mixture at that location. Heat Generation Similar to heat generation throughout the medium, some mass transfer problems involve chemical reaction that occur within the medium and result in the generation of a species throughout. The rate of generation may vary from point to point in the medium. Such reactions that occur within the medium are called homogeneous reaction. In contrast, some chemical reactions produce species at the surface, such reaction is called heterogeneous reaction and are analogous to specified surface heat flux. Convection Like heat convection, mass convection is the mass transfer mechanism between a surface and a moving fluid that involves both mass diffusion and bulk fluid motion. In mass convection, we define a concentration boundary layer in an analogous manner to the thermal boundary layer. The rate of heat convection for external flow was expressed conveniently by Newton’s law of cooling as: 𝑄̇𝑐𝑜𝑛𝑣 = ℎ𝑐𝑜𝑛𝑣 𝐴𝑠 (𝑇𝑠 − 𝑇∞ ) Likewise, the rate of mass convection can be expressed as 2 𝑚̇𝑐𝑜𝑛𝑣 = ℎ𝑚𝑎𝑠𝑠 𝐴𝑠 (𝐶𝑠 − 𝐶∞ ) Where ℎ𝑚𝑎𝑠𝑠 is the mass transfer coefficient. 𝐴𝑠 is the surface area and (𝐶𝑠 − 𝐶∞ ) denotes the concentration difference across the concentration boundary layer. 6.3 Mass Diffusion 6.3.1 Mass Concentration As it is mentioned previously and according to Fick’s law of mass diffusion, the species concentration plays the main role in mass transfer. The two following ways are commonly used in expressing species concentration: 1. Mass basis On mass basis, concentration is expressed in terms of density (or mass concentration), which is mass per unit volume. Considering a small volume V at a location within the mixture, the densities of a species (subscript i) and of the mixture (no subscript) at that location are given by: Partial density of species i : 𝜌𝑖 = 𝑚𝑖 /𝑉 Total density of mixture: 𝜌= 𝑚 𝑉 =∑ 𝑚𝑖 𝑉 = ∑ 𝜌𝑖 Mass concentration can also expressed in dimensionless form in terms of mass fraction w as 𝑚 𝑚𝑖 /𝑉 𝜌 Mass fraction of species i: 𝑤𝑖 = 𝑚𝑖 = 𝑚/𝑉 = 𝜌𝑖 As it is clear, wi varies from 0 to 1 and the conservation of mass requires that the sum of the mass fraction of constituents of mixture be equal to 1 (∑ 𝑤𝑖 = 1). 2. Mole Basis On mole basis, concentration is expressed in terms of molar concentration (molar density) as: Partial molar concentration of species i : 𝐶𝑖 = 𝑁𝑖 /𝑉 𝐶= Total molar concentration of mixture : 𝑁 𝑉 =∑ 𝑁𝑖 𝑉 = ∑ 𝐶𝑖 And dimensionless form of concentration can be expressed in terms of mole fraction y as Mole fraction species: 𝑦𝑖 = 𝑁𝑖 𝑁 = 𝑁𝑖 /𝑉 𝑁/𝑉 = 𝐶𝑖 𝐶 As it is seen, the summation of mole fraction of species is equal to 1 (∑ 𝑦𝑖 = 1) and single mole fraction yi takes a value from 0 to 1. The mass m and mole number N of a substance are related to each other by: 3 𝑚 = 𝑁 𝑀 (or for a unit volume, 𝜌 = 𝐶 𝑀), where M is the molar mass (molecular 𝜌 𝜌 weight) of substance. Accordingly; 𝑐𝑖 = 𝑀𝑖 (for species) and 𝑐 = 𝑀 (for mixture). Where 𝑖 M is the molar mass of the mixture which can be determined by: 𝑀= 𝑚 ∑ 𝑚𝑖 ∑ 𝑀𝑖 𝑁𝑖 𝑁𝑖 = = = ∑ 𝑀𝑖 = ∑ 𝑦𝑖 𝑀𝑖 𝑁 𝑁 𝑁 𝑁 The mass and mole fractions of species i of a mixture are related to each other by: 𝑤𝑖 = 𝜌𝑖 𝐶𝑖 𝑀𝑖 𝑀𝑖 = × = 𝑦𝑖 𝜌 𝐶 𝑀 𝑀 For perfect gas: The pressure fraction, is given through the following relations: 𝑃𝑖 𝑁𝑖 𝑅𝑢 𝑇/𝑉 𝑁𝑖 = = = 𝑦𝑖 𝑃 𝑁 𝑅𝑢 𝑇/𝑉 𝑁 Therefore, the pressure fraction of species i of an ideal gas mixture is equivalent to the mole fraction of that species. 6.3.2 Fick’s Law of Diffusion Stationary Medium consisting of Two Species The linear relationship between the rate of diffusion of mass and the concentration gradient proposed by Fick is known as Fick’s Law of diffusion and can be expressed as: Mass flux = Constant of proportionality × Concentration gradient has different forms, according to the expression of concentration (ρ, C, w, y), thus one can write mathematically (as shown in figure (5) : Mass basis: 𝐽𝑑𝑖𝑓𝑓,𝐴 = −𝜌 𝐷𝐴𝐵 Mole basis: 𝐽𝑑𝑖𝑓𝑓,𝐴 = −𝐶 𝐷𝐴𝐵 𝑑𝜌𝐴 /𝜌 𝑑𝑥 𝑑𝐶𝐴 /𝐶 𝑑𝑥 = −𝜌 𝐷𝐴𝐵 = −𝐶 𝐷𝐴𝐵 𝑑𝑤𝐴 (kg/m2.s) 𝑑𝑥 𝑑𝑦𝐴 𝑑𝑥 (kmole/m2.s) For the special case, when ρ and C can be assumed constant, the foregoing equations of diffusive mass flux and diffusive molar flux take simpler form as: Mass basis: Mole basis: 𝐽𝑑𝑖𝑓𝑓,𝐴 = − 𝐷𝐴𝐵 𝐽𝑑𝑖𝑓𝑓,𝐴 = − 𝐷𝐴𝐵 𝑑𝜌𝐴 𝑑𝑥 𝑑𝐶𝐴 𝑑𝑥 (kg/m2.s) (kmole/m2.s) 4 Figure (5) Various expression of Fick’s law of diffusion for a binary mixture. This assumption of ρ = constant and C = constant, is appropriate for solid and dilute solution but for gas mixture and concentrated liquids solutions, it is not true. The mass diffusivity (binary diffusion coefficient) DAB is a transport property as thermal diffusivity and momentum diffusivity (kinematic viscosity) v. The diffusion coefficients are usually determined experimentally. For dilute gases at ordinary pressure, DAB has the following relations: 𝐷𝐴𝐵 ∝ 𝑇 3/2 𝐷𝐴𝐵,1 or 𝑃 𝐷𝐴𝐵,2 𝑃 3/2 𝑇 = 𝑃2 × (𝑇1 ) 1 2 Table (1) gives the diffusion coefficient of some gases in air at 1atm pressure. The binary diffusion coefficient for several binary gas mixtures and solid and liquid solutions are given in Tables (2) and Table (3). As it is clear, from these tables, the diffusion coefficients, in general, are highest in gases and lowest in solids. Also, it is noted, that the diffusion coefficients increase with temperature. For water vapor diffuses in air, there is an empirical formula states, also table (4), that: 𝐷𝐻2 𝑜−𝐴𝑖𝑟 = 1.87 × 10−10 𝑇 2.072 (m2/s) 𝑃 280 K < T < 450 K Where P is the total pressure in atm and T is the temperature in K. 6.4 Boundary Conditions The two common types of boundary conditions are : 1. Specified species concentration, which is corresponding to specified temperature. 2. Specified species flux, which is corresponding to specified heat flux. Despite their apparent similarity, an important difference exists between temperature and concentration; temperature is necessarily a continuous function, but concentration, in general is not. For water-air interface, the concentration of air on the two sides is obviously very different as shown in figure (6).In fact the concentration of air in water is close to zero. Likewise, the concentration of water on the two sides of water air interface is also different even when air is saturated. Accordingly, in mass transfer boundary condition, not enough the location but the side of this boundary of great importance (e. g. liquid side or gas side). Figure (6) The concentration of species on the two sides of liquid-gas (or solid-gas or solidliquid)ninterface are usually not the same. Using Fick’s Law, the constant species flux boundary condition for a diffusing species A at the boundary x = 0 is: −𝐶 𝐷𝐴𝐵 𝑑𝑦𝐴 𝑑𝑥 = 𝐽𝐴,0 or 5 −𝜌 𝐷𝐴𝐵 𝑑𝑤𝐴 𝑑𝑥 = 𝐽𝐴,0 For impermeable surface, no mass can be diffused, and accordingly (see figure (7) : 𝑑𝑦𝐴 (0) 𝑑𝑤𝐴 (0) = =0 𝑑𝑥 𝑑𝑥 To apply the specified concentration boundary condition, the concentration of species at the boundary must be known. This information is usually obtained from the requirement that thermodynamics equilibrium must exist at the interface of the two phases of species. In case of air-water interface, the concentration values of water vapor in the air are easily determined from saturation data. Figure (7) An impermeable surface in mass transfer is analogous to an insulated surface in heat transfer. The situation is similar at solid-liquid interface. Again, at given temperature, only a certain amount of solid can be dissolved in liquid, and the solubility of the solid in liquid is determined from the requirement that thermodynamic equilibrium exists between the solid and the solution at the interface. The solubility represents the maximum amount of solid that can be dissolved in a liquid at specified temperature. Table (5) gives solubility data for sodium chloride (Na Cl) and Calcium bicarbonate [Ca (h CO3)2] at various temperature. For a solid diffuses in a liquid, the mass fraction of salt at the interface and at liquid side is given by: 𝑤𝑠𝑎𝑙𝑡,𝑙𝑖𝑞𝑢𝑖𝑑 𝑠𝑖𝑑𝑒 = 𝑚𝑠𝑎𝑙𝑡 𝑠𝑜𝑙𝑢𝑏𝑖𝑙𝑖𝑡𝑦 = 𝑚 𝑚𝑠𝑎𝑙𝑡 + 𝑚𝑙𝑖𝑞𝑢𝑖𝑑 Whereas the mass fraction of salt in the pure solid salt is wsalt,solid side =1.0. Most gases are weakly soluble in liquids (such as air in water) and for dilute solutions the mole fractions of a species i in the gas and liquid phases at the interface are observed to be proportional to each other. That is, yi,gas side ∝ yi,liquid side or Pi,gas side ∝ P yi, liquid side since yi, gas side = Pi,gas side / P for ideal gas mixtures. This is known as Henry’s law and is expressed as: 𝑦𝑖,𝑙𝑖𝑞𝑢𝑖𝑑 𝑠𝑖𝑑𝑒 = 𝑃𝑖,𝑔𝑎𝑠 𝑠𝑖𝑑𝑒 𝐻 (at interface) Where H is Henry’s constant, its values for a number of aqueous solutions are given in Table (6). as it is clear from the Table (6), the dissolved gases decrease with increasing temperature. Also, from equation the concentration of a gas dissolved in a liquid is proportional to the partial pressure of the gas. For highly dissolved gases in liquid, the mole fraction in liquid side can be given, according to Raoult’s law, as: 𝑃𝑖,𝑔𝑎𝑠 𝑠𝑖𝑑𝑒 = 𝑦𝑖,𝑔𝑎𝑠 𝑠𝑖𝑑𝑒 𝑃 = 𝑦𝑖,𝑙𝑖𝑞𝑢𝑖𝑑 𝑠𝑖𝑑𝑒 𝑃𝑖,𝑠𝑎𝑡 (𝑇) 6 Where Pi, sat(T) is the saturation pressure of the species i at the interface temperature and P is the total pressure on the gas phase side. Gases may also dissolve in solids. The concentration of gas species i in the solid at the interface Ci,solid side is proportional to the partial pressure of species i in the gas Pi, gas side on the gas side of the interface and is expressed as: 𝐶𝑖,𝑠𝑜𝑙𝑖𝑑 𝑠𝑖𝑑𝑒 = T × 𝑃𝑖,𝑔𝑎𝑠 𝑠𝑖𝑑𝑒 (kmole/m3) Where T is the solubility. Expressing the pressure in bars and noting that the unit of molar concentration is kmole of species i per m3, the unit of solubility is kmole/m3.bar. Solubility data for selected gas-solid combinations are given in Table (7). 7 8 9