Electronic Supplementary Material for: Morphological differentiation
advertisement

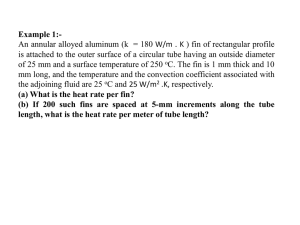
Electronic Supplementary Material for: Morphological differentiation in the damselfish Abudefduf saxatilis along the Mexican Atlantic coast is associated with environmental factors and high connectivity. Evolutionary Biology Victor J. Piñeros, Oscar Rios-Cardenas, Carla Gutiérrez-Rodríguez, Luis Mendoza-Cuenca. Corresponding author: Oscar Rios-Cardenas; Red de Biología Evolutiva, Instituto de Ecología A.C., e-mail address: oscar.rios@inecol.mx. Materials and Methods Environmental variables In accordance with our hypotheses and based on previous studies, we looked for databases that contained marine environmental variables that are known to affect fish morphology (Booth & Hixon 1999; Aguilar-Medrano et al. 2013; Fulton et al. 2013). We found data to test our hypothesis in two main sources: National Oceanic and Atmospheric Administration, NOAA (http://www.noaa.gov/ocean.html) and Atlas Climático Digital de México (http://atlasclimatico.unam.mx/atlas/mar/). Although NOAA is the most comprehensive database, there are no studies that support a direct effect of most of its available variables (i.e. salinity, dissolved oxygen, silicate, phosphate, nitrate) on the morphological traits included in our hypotheses. Therefore, we obtained environmental data from the Atlas Climático Digital de México maintained by the Centro de Ciencias de la Atmósfera from the Universidad Nacional Autónoma de México (UNAM), which includes data of all our sampling localities (i.e. reefs) at a fine scale. Environmental variables included: 1) superficial water temperature (ºC); 2) water chlorophyll-a concentration (mg/m3), as an estimator of primary productivity; and 3) geostrophic velocity (cm/s), which is a measure of sea-surface flow that takes into account wind force, earth rotation, tide force and water movement from high pressure to a low-pressure areas (Lalli & Parson 2006). Sea-surface temperature and water chlorophyll-a concentration have a spatial resolution of 4 km and are taken by the Aqua MODIS (Moderate-Resolution Imaging Spectroradiometer) 4-micron satellite sensor of the NASA, for the period between (2003-2012). Geostrophic velocity data, from the database AVISO (Archiving, Validation, and Interpretation of Satellite Oceanographic data), has a spatial resolution of 1/3º and includes a period of 18 years (1993-2012). Primers and PCR conditions for mtDNA amplification We used primers Cytb-09H (Song 1994) and Cytb-07L (Taberlet et al. 1992) to amplify the cytb; and primers K and G (Lee et al. 1995), as well as internal primers designed for this study (RC-F: 5'-GTACATATATGTAATATCACCA-3’ and RC-R: 5'GTTTTGTGGATGCATTATTATAAT-3’), to amplify the control region (CR). Cyt-b and CR PCR reactions consisted of a total volume of 15 µL with 1.5 µL of 50-100 ng DNA template, 3 µL of 5X buffer, 0.3 µL of 5 U/µL GoTaq polymerase (Promega), 0.38 µL of 8 mM dNTPs, 0.9 µL of 25 mM MgCl2, 0.3 µL of 10 mg/mL BSA and 0.3 µL of 10 µM for each oligonucleotide. The thermal cycler profiles for cyt-b consisted of an initial denaturation step at 95 °C for 60 s, followed by 35 cycles of 95 °C for 30 s, 58 °C for 45 s and 72 °C for 60 s with a final extension at 72 °C for 10 min. The CR profile included an initial denaturation step at 93 °C for 5 min, followed by 35 cycles of 93 °C for 60 s, 55 °C (for K and G primers) and 51 °C (for RC-F and RC-R primers) for 60 s and 72 °C for 2 min with a final extension at 72 °C for 10 min. PCR conditions for microsatellites amplification For the four microsatellites loci previously developed in Abudefduf luridus (Carvalho et al. 2000), the total volume of multiplex reactions was 6 µL, with 3 µL of PCR Multiplex Master Mix (Qiagen), 0.6 µL of 2 µM for each of the primers and 1 µL of 50-100 ng DNA. Thermal cycling conditions consisted of an initial denaturation step at 95 °C for 15 min, followed by 30 cycles of 94 °C for 30 s, 52 °C for 90 s and 72 °C for 60 s with a final extension at 60 °C for 30 min. Statistical power of the microsatellite markers to detect genetic differentiation We determined the statistical power of the microsatellites markers to detect significant genetic differentiation between the Gulf of Mexico and the Mexican Caribbean using the computer software POWSIM 4.1 (Ryman & Palm 2006). We simulated the sampling of 200 individuals into two populations based on a random drawing of alleles that occurred at the observed overall frequency in each marine region. We performed the simulations using different FST values and 1000 runs for each value. We determined the statistical power using the proportion of simulations for which Fisher’s exact and Chi-square tests showed a significant deviation from 0 (significant genetic differentiation; Ryman et al. 2006). References Aguilar-Medrano, R., Frédérich, B., Balart, E.F. & De Luna, E. (2013). Diversification of the pectoral fin shape in damselfishes (Perciformes, Pomacentridae) of the Eastern Pacific. Zoomorphology, 132, 197-213. Booth, D.J. & Hixon, M.A. (1999). Food ration and condition affect early survival of the coral reef damselfish, Stegastes partitus. Oecologia, 121, 364-368. Carvalho, M.C., Streiff, R., Guillemaud, T., Afonso, P., Santos, R.S. & Cancela, M.L. (2000). Isolation and characterization of polymorphic microsatellite markers in Abudefduf luridus (Pisces: Pomacentridae). Molecular Ecology, 9, 993-1011. Fulton, C.J., Binning, S.A., Wainwright, P.C. & Bellwood, D.R. (2013). Wave-induced abiotic stress shapes phenotypic diversity in a coral reef fish across a geographical cline. Coral Reefs, 32, 685-689. Lalli, C.M. & Parson, T.R. (2006). Biological Oceanography: An introduction. Oxford: Elsevier Butterworth-Heinemann.20. Lee, W., Conroy, J., Howell, W.H. & Kocher T.D. (1995). Structure and evolution of teleost mitochondrial control regions. Journal of Molecular Evolution, 41, 54-66. Ryman, N. & Palm, S. (2006). POWSIM: a computer program for assessing statistical power when testing for genetic differentiation. Molecular Ecology Notes, 6, 600-602. Ryman, N., Palm, S., André, C., Carvalho, G.R., Dahlgren, T.G., Jorde, P.E., Laikre, L., Larsson, L.C., Palmé, A. & Ruzzante, D.E. (2006). Power for detecting genetic divergence: differences between statistical methods and marker loci. Molecular Ecology, 15, 2031-2045. Song, C. (1994). Molecular evolution of the cytochrome b gene among Percid fishes. PhD thesis. Champaing. University of Illinois. Taberlet, P., Meyer, A. & Bouvet, J. (1992). Unusual mitochondrial DNA polymorphism in two local populations of blue tit (Parus caerulens). Molecular Ecology, 1, 27-36. Table S1 Morphometric pairwise comparisons using Goodall’s F with 2,500 bootstrap replicates and BGPCA with 1,000 bootstrap replicates of body and head measurements of Abudefduf saxatilis collected on seven reefs. P values shown in bold indicate significant differences. For site abbreviations refer to Table 1. Body Shape Head Shape Goodall’s Reef LI vs TX LI vs VE LI vs AL LI vs CI LI vs XC LI vs CYI TX vs VE TX vs AL TX vs CI TX vs XC TX vs CYI VE vs AL VE vs CI VE vs XC VE vs CYI AL vs CI AL vs XC AL vs CYI CI vs XC CI vs CYI XC vs CYI F 0.51 1.14 0.44 1.99 1.38 1.61 3.1 1.89 4.69 3.17 4.5 1.08 2.15 1.81 1.12 2.13 1.77 1.76 1.07 0.89 1.19 d.f. 18, 288 18, 216 18, 378 18, 216 18, 162 18, 216 18, 360 18, 522 18, 360 18, 306 18, 360 18, 450 18, 288 18, 234 18, 288 18, 450 18, 396 18, 450 18, 234 18, 288 18, 234 P 0.9523 0.3135 0.9782 0.0112 0.1464 0.0586 < 0.00001 0.0149 < 0.00001 < 0.00001 < 0.00001 0.3645 0.0048 0.0255 0.3272 0.0044 0.0268 0.0279 0.3794 0.5944 0.2741 Bootstrap Procrustes F P 0.51 0.8164 1.14 0.3228 0.44 0.8812 1.99 0.0896 1.38 0.246 1.61 0.1588 3.1 0.0176 1.89 0.0808 4.69 0.0004 3.17 0.0084 4.5 0.0016 1.08 0.372 2.15 0.0556 1.81 0.124 1.12 0.3204 2.13 0.0456 1.77 0.0912 1.76 0.1012 1.07 0.3572 0.89 0.45 1.19 0.2872 BGPCA Goodall’s P 0.82 0.2 0.53 0.06 0.09 0.07 0.01 0.02 0.01 0.01 0.01 0.39 0.03 0.05 0.4 0.04 0.05 0.15 0.53 0.77 0.3 F 0.35 3.16 2.25 2.17 3.13 2.93 5.2 4.13 3.91 4.39 4.59 2.21 2.78 2.02 0.97 2.11 2.06 1.3 1.33 1.18 1.18 d.f. 26, 364 26, 312 26, 520 26, 286 26, 208 26, 338 26, 520 26, 728 26, 494 26, 416 26, 546 26, 676 26, 442 26, 364 26, 494 26, 650 26, 572 26, 702 26, 338 26, 468 26, 390 P 0.999 < 0.00001 0.000449 0.0012 < 0.00001 < 0.00001 < 0.00001 < 0.00001 < 0.00001 < 0.00001 < 0.00001 0.000554 < 0.00001 0.0026 0.5102 0.001145 0.00169 0.1432 0.13351 0.24613 0.2529 Bootstrap Procrustes F P 0.35 0.928 3.18 0.028 2.29 0.0484 2.15 0.086 3.14 0.0372 2.95 0.0208 5.23 0.0008 4.15 0.0004 3.95 0.004 4.44 0.0032 4.62 0.0012 2.2 0.045 2.74 0.0316 2.04 0.0724 1.01 0.416 2.05 0.0596 2.08 0.0672 1.35 0.2216 1.34 0.244 1.12 0.3452 1.14 0.3416 BGPCA P 0.97 0.03 0.12 0.05 0.01 0.01 0.01 0.01 0.01 0.01 0.01 0.05 0.01 0.07 0.063 0.03 0.11 0.41 0.17 0.25 0.44 Table S2 Summary of microsatellite diversity of Abudefduf saxatilis for each of the 12 loci at each reef and reef system. We show number of analyzed individuals (N), number of alleles (AN), allelic richness (AR), private allelic richness (AP) observed (HO) and expected (HE) heterozygosity, inbreeding coefficient (FIS), and frequency of null alleles (FNA) for loci that deviated from HWE due to heterozygote deficits. Abbreviations correspond to those in Table 1. Reef/Reef system LI TX VE AL N AN AR AP HO HE FIS FNA N AN AR AP HO HE FIS FNA N AN AR AP HO HE FIS N AN AR Absa1 Absa10 Absa17 Absa18 Absa23 Absa30 Absa38 Absa41 2al10 2al13 2al2 2al24 29 19 13.5 2.40 0.79 0.93 0.16 16 12 11.1 0.76 0.94 0.88 -0.03 12 10 10.0 1.00 1 0.85 -0.14 19 14 11.1 29 19 12.9 0.86 0.97 0.92 -0.04 16 13 11.4 0.96 0.81 0.88 0.11 12 13 13.0 1.07 1 0.9 -0.07 19 17 14.0 29 16 11.3 0.21 0.55 0.89 0.4 0.18 16 13 11.6 0.05 0.69 0.9 0.26 12 13 13.0 1.59 0.75 0.91 0.21 19 11 10.1 29 21 14.4 0.53 0.9 0.93 0.06 16 19 15.9 0.12 1 0.93 -0.05 12 18 18.0 0.10 1 0.93 -0.04 19 19 14.8 29 4 2.8 0.00 0.41 0.4 -0.02 16 4 3.5 0.90 0.63 0.53 -0.15 12 3 3.0 0.06 0.42 0.54 0.27 19 2 2.0 29 17 11.7 0.42 0.86 0.91 0.07 16 14 12.3 1.16 0.69 0.89 0.26 0.11 12 11 11.0 0.03 1 0.88 -0.09 19 15 12.7 29 13 10.3 0.20 0.79 0.89 0.12 16 14 12.9 0.63 0.94 0.92 0.01 12 12 12.0 0.24 0.83 0.88 0.1 19 14 11.3 29 13 9.9 0.45 0.9 0.88 0 16 13 11.6 0.90 0.81 0.89 0.12 12 12 12.0 0.00 1 0.9 -0.07 19 14 11.3 29 16 11.6 0.12 0.97 0.9 -0.05 16 13 11.4 0.32 0.94 0.89 -0.02 12 12 12.0 0.08 1 0.89 -0.08 19 14 11.9 29 4 3.7 0.00 0.66 0.64 0 16 5 4.5 0.75 0.38 0.46 0.21 12 4 4.0 0.00 0.67 0.65 0.02 19 5 4.6 29 18 13.4 0.71 0.93 0.93 0.01 16 16 13.5 0.53 0.88 0.9 0.06 12 10 10.0 0.09 0.83 0.86 0.08 19 15 12.4 29 13 10.4 0.42 0.48 0.89 0.47 0.21 16 13 11.4 0.87 0.75 0.89 0.19 12 13 13.0 1.05 0.92 0.89 0.01 19 13 9.9 CYI CI XC TRS VRS AP HO HE FIS FNA N AN AR AP HO HE FIS N AN AR AP HO HE FIS N AN AR AP HO HE FIS FNA N AN AR AP HO HE FIS FNA N 0.89 0.89 0.86 -0.02 13 13 12.6 0.11 1 0.9 -0.07 18 13 11.1 0.02 0.83 0.89 0.09 15 13 11.4 0.03 0.93 0.88 -0.03 45 21 19.0 4.44 0.84 0.92 0.10 31 0.80 0.95 0.93 0.01 13 11 10.7 0.93 0.92 0.88 -0.01 18 17 14.4 0.15 1 0.92 -0.05 15 14 12.7 0.39 1 0.9 -0.07 45 20 18.4 2.46 0.91 0.92 0.02 31 0.70 0.68 0.89 0.26 13 9 8.8 0.00 0.92 0.87 -0.03 18 10 9.6 0.00 0.83 0.88 0.08 15 10 9.3 0.07 0.53 0.86 0.41 0.17 45 16 15.2 0.32 0.60 0.91 0.35 0.16 31 0.21 0.95 0.91 -0.01 13 12 11.6 0.99 1 0.89 -0.08 18 15 12.7 0.68 0.83 0.9 0.11 15 14 12.6 0.07 0.87 0.89 0.06 45 20 17.6 1.72 0.80 0.92 0.14 31 0.05 0.95 0.93 0 13 18 17.1 1.04 0.85 0.93 0.13 18 19 14.9 0.75 0.94 0.92 0 15 18 15.8 0.06 0.8 0.93 0.17 45 24 21.9 0.89 0.93 0.94 0.02 31 1.42 0.58 0.89 0.37 0.16 13 10 9.7 0.54 0.62 0.87 0.33 18 10 9.6 0.01 0.72 0.89 0.21 15 10 9.0 0.00 0.73 0.83 0.15 45 16 15.1 1.30 0.84 0.91 0.08 31 1.47 0.89 0.87 -0.01 13 9 8.8 0.12 0.92 0.83 -0.07 18 15 12.3 1.14 0.94 0.89 -0.04 15 9 8.6 1.07 1 0.85 -0.14 45 15 14.0 1.67 0.87 0.90 0.04 31 0.11 0.79 0.89 0.14 13 11 10.8 0.92 0.92 0.89 0 18 13 11.6 0.90 0.94 0.9 -0.02 15 15 13.6 1.54 1 0.92 -0.06 45 17 15.5 0.45 0.96 0.91 -0.04 31 0.63 0.68 0.7 0.05 13 3 3.0 0.00 0.46 0.6 0.27 18 4 3.7 0.00 0.67 0.6 -0.08 15 3 3.0 0.00 0.4 0.5 0.23 45 5 4.7 0.69 0.56 0.60 0.08 31 0.80 0.95 0.91 -0.02 13 13 12.5 1.02 0.92 0.89 0 18 16 13.1 0.88 0.94 0.91 -0.01 15 13 11.7 0.16 0.87 0.87 0.03 45 21 19.3 1.23 0.91 0.93 0.03 31 0.24 0.74 0.85 0.16 13 14 13.5 2.11 1 0.91 -0.06 18 12 10.8 0.01 0.78 0.89 0.15 15 9 8.4 0.03 0.73 0.82 0.14 45 17 15.1 1.38 0.58 0.90 0.37 0.17 31 0.00 0.37 0.36 0.01 13 4 3.8 0.96 0.46 0.54 0.19 18 4 3.6 0.01 0.39 0.44 0.14 15 4 3.6 0.21 0.67 0.54 -0.2 45 6 4.8 0.94 0.49 0.45 0.07 31 MRS AN AR AP HO HE FIS FNA N AN AR AP HO HE 16 16.0 2.01 0.94 0.87 -0.06 46 15 14.1 0.08 0.91 0.90 FIS 0.00 FNA - 19 19.0 2.54 0.97 0.93 -0.03 46 20 18.3 0.74 0.98 0.93 16 16.0 1.72 0.71 0.92 0.24 0.11 46 13 12.4 0.02 0.76 0.89 16 16.0 0.55 0.97 0.91 -0.05 46 19 17.1 1.46 0.89 0.92 22 22.0 0.55 0.97 0.94 -0.02 46 26 23.9 1.74 0.87 0.95 16 16.0 2.73 0.68 0.89 0.26 46 14 12.6 0.23 0.70 0.88 16 16.0 2.38 0.94 0.89 -0.04 46 18 15.9 2.31 0.96 0.88 16 5.0 0.33 0.87 0.91 0.05 46 19 17.0 2.66 0.96 0.92 5 18.0 1.01 0.68 0.68 0.02 46 4 3.7 0.00 0.52 0.57 18 16.0 1.40 0.90 0.91 0.02 46 20 18.3 1.67 0.91 0.92 16 16.0 1.55 0.81 0.89 0.11 46 17 15.4 2.54 0.83 0.89 3 3.0 0.03 0.39 0.44 0.14 46 6 5.2 1.18 0.50 0.51 -0.04 - 0.16 0.07 0.04 - 0.09 - 0.22 0.1 -0.07 - -0.03 - 0.10 - 0.02 - 0.09 - 0.04 - FIS values in bold indicate significant deviations from HWE after Bonferroni corrections (α’ = 0.002 for reef; α’ = 0.004 for reef system). Fig. S1 Anatomical landmarks used in the geometric morphometric analysis of a Body shape: (1) rostrum, (2) rostral insertion of the dorsal fin, (3) rostral insertion of the pectoral fin, (4) caudal insertion of the pectoral fin, (5) rostral insertion of the pelvic fin, (6) rostral insertion of the anal fin, (7) limit between the soft and spiny dorsal fin, (8) caudal insertion of the dorsal fin, (9) dorsal insertion of the caudal fin, (10) caudal insertion of the anal fin, (11) ventral insertion of the caudal fin; b Head shape: (1) rostral extremity of the premaxilla, (2) nostril, (3-6) ventral, rostral, dorsal and caudal margins of the eye, (7) center of the eye, (8) dorsal tip of the preopercular, (9) dorsal edge of the operculum, (10) ventral edge of the operculum, (11) insertion of the operculum along the body profile, (12) posteroventral corner of the operculum, (13-14) rostral and caudal edge of the lower jaw teeth, (15) caudal extremity of the premaxilla; and c standard length (sl) of Abudefduf saxatilis. Fig. S2 Statistical power to detect significant population genetic differentiation as a function of FST. Statistical power was determined as the proportion simulations for which a Chi-square and b Fisher’s exact tests showed a significant deviation from 0 (significant genetic differentiation). FST values as low as 0.005 can be detected with both tests at probabilities of 99% with the number of loci and sample sizes that we used.