POD 1 Define 2 words. Read the article. Analyze the images
advertisement

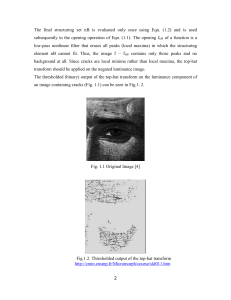
POD 1 Define 2 words. Read the article. Analyze the images. ANSWER ? VOCABULARY Inference Observation Author Affiliations Conditions for crack propagation by frost wedging . THOMAS M. THARP1 + . 1Department of Earth and Atmospheric Sciences, Purdue University, West Lafayette, Indiana 47907 . Abstract Susceptibility to frost wedging is influenced by rock properties and environmental parameters governing stress intensity at crack tips. The ice pressure causing frost wedging arises from expansion of water upon freezing, or from the adsorptive force that draws water into the interface between ice and rock. Ice pressure is an order of magnitude higher when ice and water pressures remain equal during freezing (confined water) than when free water remains at atmospheric pressure (unconfined water) and adsorptive force conditions prevail. Capillarity generally does not produce stresses of sufficient magnitude to propagate cracks. Two distinct crack geometries are analyzed. In coarse-grained porous sedimentary rock, the diameter of pores dictates effective crack aperture and a crack with effectively constant aperture (rectangular crack) results. In other rocks, the aperture of cracks diminishes gradually toward the crack tip (tapered crack). Rectangular cracks more than ∼1 mm long will propagate for short-term confined-water conditions, or with stress corrosion, for long-term unconfined-water conditions. The tapered cracks of crystalline rock must be 14 cm long to propagate under short-term confined-water freezing and 1.4 cm long for long-term loading with unconfined-water conditions. Tapered cracks an order of magnitude shorter will propagate with unconfined water if they are connected to a reservoir of unfrozen water, allowing adsorptive force suction. Cracks with aspect ratio (maximum aperture/crack length) greater than 0.01 and terminating in sharp crack tips are most susceptible to frost wedging, because ice expansion in these wide cracks causes sufficient dilation to produce critical stress intensity at the crack tip. Some relaxation of ice pressure by plastic flow is expected in wide cracks, but its effect is small. QUESTION: Using inference, which weathering process resulted in the erosion from image A to image B? (CIRCLE ONE LETTER) A Frozen water acts as a solute. B Water expands when it freezes. C. D. The mass of water increases when it freezes. Frozen water dissolves most types of rocks POD 2 Verify Materials. Define 2 words. Conduct experiment. Analyze data. ANSWER ? MATERIALS Salt, Water colder and warmer, thermometer, triple beam balance Vocabulary Solute Solvent PROCEDURE: 1. Measure 1 gram solute. (salt NaCl) 2. Record temperatures of solvent A_______ and B _________(water) 3. Stir to dissolve the 1 gram of solute into solvent 4. Repeat steps 1-3 until the solute will not dissolve completely into the solvent 5. Record the number of grams of solute that completely dissolved A_______AND B______ QUESTION: Which graph best represents the results of the experiment? (CIRCLE ONE LETTER) POD 3 Verify Materials. Define 1 word. Conduct experiment. Analyze data. ANSWER ?s MATERIALS M&Ms, Water colder and warmer, thermometer, magnifying lense Vocabulary Diffuse PROCEDURE: 1. Record temperatures of solvent A_______ and B _________(water) 2. Place same colored solute (M&M coating) into A & B 3. Repeat 5 trials (10 M&Ms) Record of diffusion by recording tallies for fastest/first temperature to show diffusion of solute into solvent (when the coating is in the water) A_________________ B_________________ QUESTIONS: What is the relationship between diffusion rate and temperature of solvent? (CIRCLE ONE LETTER) A. B. C. D. E. As temperature of solvent decreases the rate of diffusion decreases. As temperature of solvent increases the rate of diffusion increases. As temperature of solvent decreases the rate of diffusion increases. As temperature of solvent increases the rate of diffusion decreases. QUESTION: Which image shows diffusing? _______Which image shows dissolving?_______ Diffbtwn. Difference between osmosis and diffusion Posted on November 23, 2009 Sciencephotolibrary.com A B