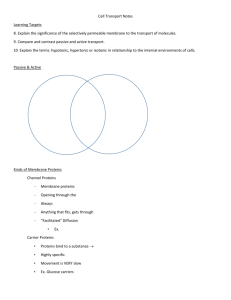
Chapter 2.5 - 2.10 Outline - Heartland Community College
advertisement

CHAPTER 2 BASIC CHEMISTRY & MOLECULES OF LIFE Living organisms and non-living substances are composed of matter. The basic unit of matter is the atom. This chapter presents a study of atomic structure, which allows us to see how atoms interact by ionic or covalent bonds to form molecules. All living things are composed of 70-90% water; as such, a thorough knowledge of the chemical and physical properties of water, as well as those properties of acids and bases, is critical to understanding the chemistry of life. The chemical and physical properties of water are presented in detail, as is the concept of acids and bases. Organic molecules are a diverse group of chemicals that contain carbon and hydrogen bonded to other atoms. In the living organism, four type of organic molecules, or biomolecules, exist: carbohydrates, lipids, proteins, and nucleic acids. The unique chemistry of carbon make these biomolecules highly diverse in structure and function. This chapter presents a detailed description of these biomolecules and of the macromolecules built from them; various types of chemical reactions involving these organic substances are described. Many examples are presented to illustrate the diversity and biological importance of these molecules of life. Chapter 2.1 - 2.4 Outline 2.1 Chemical Elements 1. Matter is defined as anything that takes up space and has mass. 2. Matter exists in three states: solid, liquid, and gas. 3. All matter (both living and non-living) is composed of 92 naturally-occurring elements. 4. Elements, by definition, cannot be broken down to simpler substances with different chemical or physical properties. 5. Six elements (carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur—acronym CHNOPS) make up 98% of the body weight of organisms. A. Atomic Structure 1. Elements consist of tiny particles called atoms. 2. An atom is the smallest unit of an element that displays the properties of the element. 3. One or two letters (e.g., H, Na) create the atomic symbol of the element. 4. The atomic mass of an atom depends on the presence of certain subatomic particles. a. Atoms contain specific numbers of protons, neutrons, and electrons. b. Protons and neutrons are in the nucleus of an atom; electrons move around the nucleus. c. Protons are positively charged particles; neutrons have no charge; both have 1 atomic mass unit (amu) of weight. d. Electrons are negatively charged particles located in orbitals outside the nucleus. 5. All atoms of an element have the same number of protons, called the atomic number of the element. B. The Periodic Table 1. The periodic table shows how various characteristics of atoms of elements recur. 2. Groups are the vertical columns in the table, periods are the horizontal rows; atomic mass increases as you move down a group or across a period. 3. The atomic number is above the atomic symbol and the atomic mass is below the atomic symbol. C. Isotopes 1. Isotopes are atoms of the same element that differ in the number of neutrons (and therefore have different atomic masses). For example, carbon-12 has 6 protons and 6 neutrons, carbon-14 has 6 protons and 8 neutrons. 2. A carbon atom with 8 rather than 6 neutrons is unstable; it releases energy and subatomic particles and is thus a radioactive isotope. 19 2.2 Elements and Compounds 1. When atoms of two or more different elements bond together, they form a compound (e.g., H2O). 2. A molecule is the smallest part of a compound that has the properties of the compound. 3. Electrons possess energy, and bonds that exist between atoms in molecules therefore contain energy. A. Ionic Bonding 1. An ionic bond forms when electrons are transferred from one atom to another atom. 2. By losing or gaining electrons, atoms fill outer shells, and are more stable (the octet rule). 3. Example: sodium loses an electron and therefore has a positive charge; chlorine gains an electron to give it a negative charge. Such charged particles are called ions. 4. Attraction of oppositely charged ions holds the two atoms together in an ionic bond. 5. A salt (e.g., NaCl) is an example of an ionically-bonded compound. B. Covalent Bonding 1. Covalent bonds result when two atoms share electrons so each atom has an octet of electrons in the outer shell (or, in the case of hydrogen, 2 electrons). 2. Hydrogen can give up an electron to become a hydrogen ion (H +) or share an electron with another atom to complete its shell with 2 electrons. 3. The structural formula of a compound indicates a shared pair of electrons by a line between the two atoms; e.g., single covalent bond (H–H), double covalent bond (O=O), and triple covalent bond (N = N). Each line between the atoms represents a pair of electrons. 4. The three-dimensional shapes of molecules are not represented by structural formulas, but shape is critical in understanding the biological action of molecules. Different molecules have different threedimensional shapes, depending on the number of atoms in the molecule and the types of bonds (single , double, or triple covalent). C. Nonpolar and Polar Covalent Bonds 1. In nonpolar covalent bonds, sharing of electrons is equal, i.e., the electrons are not attracted to either atom to a greater degree. 2. With polar covalent bonds, the sharing of electrons is unequal. a. b. In a water molecule (H2O), sharing of electrons by oxygen and hydrogen is not equal; the oxygen atom with more protons attracts the electrons closer to it, and thus dominates the H 2O association. Attraction of an atom for electrons in a covalent bond is called the electronegativity of the atom; an oxygen atom is more electronegative than a hydrogen atom. c. Oxygen in a water molecule, more attracted to the electron pair, assumes a partial negative charge. D. Hydrogen Bonding 1. A hydrogen bond is a weak attractive force between the slightly positive charge of the hydrogen atom of one molecule and slightly negative charge of another atom (e.g., oxygen, nitrogen) in another or the same molecule. 2. Many hydrogen bonds taken together are relatively strong. 3. Hydrogen bonds between and within complex biological molecules (e.g., DNA, proteins) help maintain their proper structure and function. 2.3. Chemistry of Water 1. All living things are 70–90% water. 2. Because water is a polar molecule, water molecules are hydrogen bonded to one other. 3. Because of hydrogen bonding, water is liquid between 0º C and 100 o C which is essential for the existence of life. A. Properties of Water 1. Water has a high heat capacity a. The temperature of liquid water rises and falls more slowly than that of most other liquids. 20 b. A calorie is the amount of heat energy required to raise the temperature of one gram of water 1º C. c. Because the hydrogen bonds between water molecules hold more heat, water’s temperature falls more slowly than other liquids; this protects organisms from rapid temperature changes and helps them maintain homeostatic temperature. 2. Water has a high heat of vaporization. a. Hydrogen bonds between water molecules require a relatively large amount of heat to break. b. This property moderates Earth’s surface temperature; permits living systems to exist. c. When animals sweat, evaporation of the sweat removes body heat, thus cooling the animal. 3. Water is a solvent. a. Water dissolves a great number of substances (e.g., salts, large polar molecules). b. Ionized or polar molecules attracted to water are hydrophilic (“water loving”). c. Nonionized and nonpolar molecules that cannot attract water are hydrophobic (“water fearing”). d. A solution contains dissolved substances called solutes. 4. Water molecules are cohesive and adhesive. a. Cohesion allows water to flow freely without molecules separating. b. Adhesion is ability to adhere to polar surfaces; water molecules have positive and negative poles. c. Water rises up a tree from roots to leaves through small tubes. 1) Adhesion of water to walls of vessels prevents water column from breaking apart. 2) Cohesion allows evaporation from leaves to pull water column from roots. 5. Water has a high surface tension. a. Water is relatively difficult to break through at its surface. b. This property permits a rock to be skipped across a pond surface, and supports insects walking on surface. C. Acids and Bases 1. When water ionizes or dissociates, it releases a small (10 7 moles/liter) but equal number of hydrogen (H+) ions and hydroxide (OH-) ions; H – O –H → H+ + OH-. 2. Acid molecules dissociate in water, releasing hydrogen (H +) ions: HCl → H+ + Cl-. 3. Bases are molecules that take up hydrogen ions or release hydroxide ions. NaOH → Na + + OH-. 4. The pH scale indicates acidity and basicity (alkalinity) of a solution. a. pH is the measurement of free hydrogen ions, expressed as a negative logarithm of the H + concentration (-log [H+]). b. pH values range from 0 (100 moles/liter; most acidic) to 14 (1014 moles/liter; most basic). 1) One mole of water has 107 moles/liter of hydrogen ions; therefore, has neutral pH of 7. 2) An acid is a substance with pH less than 7; a base is a substance with pH greater than 7. 3) Because it is a logarithmic scale, each lower unit has 10 times the amount of hydrogen ions as next higher pH unit; as move up pH scale, each unit has 10 times the basicity of previous unit. 5. Buffers keep pH steady and within normal limits in living organisms.. a. Buffers stabilize pH of a solution by taking up excess hydrogen (H +) or hydroxide (OH-) ions. b. Carbonic acid helps keep blood pH within normal limits: H 2CO3 21 → H+ + HCO3-. MOLECULES OF LIFE Chapter 2.5 - 2.10 Outline I Organic Molecules Organic molecules contain carbon and hydrogen atoms bonded to other atoms. 1. Four types of organic molecules (biomolecules) exist in organisms: carbohydrates, lipids, proteins, and nucleic acids. 2. Organic molecules are a diverse group; even a simple bacterial cell contains some 5,000 organic molecules. A. The Carbon Atom 1. The chemictry of the carbon atom allows it to form covalent bonds with as many as four other elements (generally with the CHNOPS elements). 2. Hydrocarbons are chains of carbon atoms bonded exclusively to hydrogen atoms; hydrocarbons can be branched and they can form ringed (cyclic) compounds. 3. Carbon atoms can form double or triple bonds with certain atoms (carbon, nitrogen). B. The Carbon Skeleton and Functional Groups 1. The carbon chain of an organic molecule is called its skeleton or backbone. 2. Functional groups are clusters of specific atoms bonded to the carbon skeleton with characteristic structure and functions. a. As an example, the addition of an –OH (hydroxyl group) to a carbon skeleton turns the molecule into an alcohol. b. Ethyl alcohol (ethanol) is hydrophilic (dissolves in water) because the hydroxyl group is polar. c. Nonpolar organic molecules are hydrophobic (cannot dissolve in water) unless they contain a polar functional group. An example is ethane. d. Depending on its functional groups, an organic molecule may be both acidic and hydrophilic. An example is a hydrocarbon that contains a carboxyl group; carboxyl groups ionize in solution by releasing hydrogen ions, becoming both polar and acidic. e. Because cells are 70–90% water, the degree to which an organic molecule interacts with water affects its function. 3. Isomers are molecules with identical molecular formulas but different arrangements of their atoms (e.g., glyceraldehyde and dihydroxyacetone). C. The Macromolecules of Cells 1. Carbohydrates, lipids, proteins, and nucleic acids are called macromolecules because of their large size. 2. The largest macromolecules are called polymers, constructed by linking many of the same type of small subunits, called monomers. Examples: amino acids (monomers) are linked to form a protein (polymer); many nucleotides (monomers) are linked to form a nucleic acid (polymer). 3. Cellular enzymes carry out dehydration reactions to synthesize macromolecules. In a dehydration reaction, a water molecule is removed and a covalent bond is made between two atoms of the monomers. a. In a dehydration reaction, a hydroxyl (— OH) group is removed from one monomer and a hydrogen (— H) is removed from the other. 22 b. This produces water, and, because the water is leaving the monomers, it is a dehydration reaction. Hydrolysis (“water breaking”) reactions break down polymers in reverse of dehydration; a hydroxyl (— OH) group from water attaches to one monomer and hydrogen (— H) attaches to the other. 5. Enzymes are molecules that speed up chemical reactions by bringing reactants together; an enzyme may even participate in the reaction but is not changed by the reaction. II Carbohydrates A. Monosaccharides: Ready Energy 1. Monosaccharides are simple sugars with a backbone of 3 to 7 carbon atoms. a. Most monosaccharides of organisms have 6 carbons (hexose). b. Glucose and fructose are hexoses, but are isomers of one another; each has the formula (C 6H12O6) but they differ in arrangement of the atoms. c. Glucose is found in the blood of animals; it is the source of biochemical energy (ATP) in nearly all organisms. 2. Ribose and deoxyribose are five-carbon sugars (pentoses); they contribute to the backbones of RNA and DNA, respectively. B. Disaccharides: Varied Uses 1. Disaccharides contain two monosaccharides joined by a dehydration reaction. 2. Lactose is composed of galactose and glucose and is found in milk. 3. Maltose is composed of two glucose molecules; it forms in the digestive tract of humans during starch digestion. 4. Sucrose (table sugar) is composed of glucose and fructose; it is used to sweeten food for human consumption. C. Polysaccharides as Energy Storage Molecules 1. Polysaccharides are polymers of monosaccharides. They are not soluble in water and do not pass through the plasma membrane of the cell. 2. Starch, found in many plants, is a straight chain of glucose molecules with relatively few side branches. Amylose and amylopectin are the two forms of starch found in plants. 3. Glycogen is a highly branched polymer of glucose with many side branches. It is the storage form of glucose in animals. D. Polysaccharides as Structural Molecules 1. Cellulose is a polymer of glucose which forms microfibrils, the primary constituent of plant cell walls. a. Cotton is nearly pure cellulose. b. Cellulose is indigestible by humans due to the unique bond between glucose molecules. c. Grazing animals can digest cellulose due to special stomachs and bacteria. d. Cellulose is the most abundant organic molecule on Earth. 2. Chitin is a polymer of glucose with an amino group attached to each glucose. a. Chitin is the primary constituent of the exoskeleton of crabs and related animals (lobsters, insects, etc.). b. Chitin is not digestible by humans. III Lipids Lipids are varied in structure. 1. Lipids are hydrocarbons that are insoluble in water because they lack polar groups. 2. Fat provides insulation and energy storage in animals. 3. Phospholipids form plasma membranes and steroids are important cell messengers. 4. Waxes have protective functions in many organisms. A. Triglycerides: Long-Term Energy Storage 1. Fats and oils contain two molecular units: glycerol and fatty acids. 2. Glycerol is a water-soluble compound with three hydroxyl groups. 3. Triglycerides are glycerol joined to three fatty acids by dehydration reactions. 4. A fatty acid is a long hydrocarbon chain with a carboxyl (acid) group at one end. a. Most fatty acids in cells contain 16 to 18 carbon atoms per molecule. b. Saturated fatty acids have no double bonds between their carbon atoms. c. Unsaturated fatty acids have double bonds in the carbon chain where there are less than two hydrogens per carbon atom. 4. 23 5. 6. 7. Fats contain saturated fatty acids and are solid at room temperature (e.g., butter). Oils contain unsaturated fatty acids and are liquid at room temperature. Animals use fat rather than glycogen for long-term energy storage; fat stores more energy. IV Proteins Protein Functions 1. Support proteins include keratin, which makes up hair and nails, and collagen fibers, which support many of the body’s structures (e.g., ligaments, tendons, skin). 2. Enzymes are proteins that act as organic catalysts to accelerate chemical reactions within cells. 3. Transport functions include channel and carrier proteins in the plasma membrane, and hemoglobin that transports oxygen in red blood cells. 4. Defense functions include antibodies that prevent infection. 5. Hormones are regulatory proteins that influence the metabolism of cells. For example, insulin regulates glucose content of blood and within cells. 6. Motion within cells and by muscle contraction is provided by the proteins myosin and actin. A. Amino Acids: Building Blocks of Proteins 1. Amino acids contain an acidic group (— COOH) and an amino group (—NH2). 2. Amino acids differ according to their particular R group, ranging from single hydrogen to complicated ring compounds. 3. The R group of amino acid cystine ends with a sulfhydryl (— SH) that serves to connect one chain of amino acids to another by a disulfide bond (— S— S—). 4. There are 20 different amino acids commonly found in cells. B. Peptides 1. A peptide bond is a covalent bond between two amino acids. 2. Atoms of a peptide bond share electrons unevenly (oxygen is more electronegative than nitrogen). 3. The polarity of the peptide bond permits hydrogen bonding between different amino acids in a polypeptide. 4. A peptide is two or more amino acids bonded together. 5. Polypeptides are chains of many amino acids joined by peptide bonds. 6. A protein may contain more than one polypeptide chain; it can thus have a very large number of amino acids. a. The three-dimensional shape of a protein is critical; an abnormal sequence will have the wrong shape and will not function normally. b. Frederick Sanger determined the first protein sequence (of the hormone insulin) in 1953. C. Shape of Proteins 1. Protein shape determines the function of the protein in the organism; proteins can have up to four levels of structure (but not all proteins have four levels). 2. The primary structure is the protein’s own particular sequence of amino acids. a. Just as the English alphabet contains 26 letters, 20 amino acids can join to form a huge variety of “words.” 3. The secondary structure results when a polypeptide coils or folds in a particular way. a. The (alpha) helix was the first pattern discovered. 1) In a peptide bond, oxygen is partially negative, hydrogen is partially positive. 2) This allows for hydrogen bonding between the C=O of one amino acid and the N—H of another. 3) Hydrogen bonding between every fourth amino acid holds the spiral shape of an helix. b. The (beta) sheet was the second pattern discovered. 1) Pleated sheet polypeptides turn back upon themselves. 2) Hydrogen bonding occurs between extended lengths. c. Fibrous proteins (e.g. keratin) are structural proteins with helices and/or pleated sheets that hydrogen bond to one another. 4. Tertiary structure results when proteins are folded, giving rise to the final three-dimensional shape of the protein. This is due to interactions among the R groups of the constituent amino acids. a. Globular proteins tend to ball up into rounded shapes. 24 b. Strong disulfide linkages maintain the tertiary shape; hydrogen, ionic, and covalent bonds also contribute. 5. Quaternary structure results when two or more polypeptides combine. a. Hemoglobin is globular protein with a quaternary structure of four polypeptides; each polypeptide has a primary, secondary, and tertiary structure. D. Protein Folding Diseases 1. As proteins are synthesized, chaperone proteins help them fold into their correct shapes; chaperone proteins may also correct misfolding of a new protein and prevent them from making incorrect shapes. 2. Certain diseases (e.g., the transmissible spongiform encephalopathies, or TSEs) are likely due to misfolded proteins, called prions. V Nucleic Acids 1. Nucleic acids are polymers of nucleotides with very specific functions in cells. 2. DNA (deoxyribonucleic acid) stores the genetic code for its own replication and for the amino acid sequences in proteins. 3. RNA (ribonucleic acid) allows for translation of the genetic code of DNA into the amino acid sequence of proteins; other functions for RNA in the cell exist. 4. Some nucleotides have independent metabolic functions in cells. a. Coenzymes are molecules which facilitate enzymatic reactions. b. ATP (adenosine triphosphate) is a nucleotide used to supply energy for synthetic reactions and other energy-requiring metabolic activities in the cell 25 CHAPTER 3 CELL STRUCTURE AND FUNCTION This chapter presents an overview of the cell theory. A description of the various types of microscopes and their uses precedes a detailed study of cell structure and function. The organelles and their activities are discussed for both prokaryotic and eukaryotic cells. Various human diseases associated with organellar dysfunction are mentioned. Chapter Outline I Cellular Level of Organization 1. Detailed study of the cell began in the 1830s; some of the scientists contributing to the understanding of cell structure and function were Robert Brown, Matthais Schleiden, Theodor Schwann, and Rudolph Virchow. 2. The cell theory states that all organisms are composed of cells, that cells are the structural and functional unit of organisms, and that cells come only from preexisting cells. A. Cell Size 1. Cells range in size from one millimeter down to one micrometer. 2. Cells need a surface area of plasma membrane large enough to adequately exchange materials. 3. The surface-area-to-volume ratio requires that cells be small. a. As cells get larger in volume, surface area relative to volume decreases. b. Size limits how large the actively metabolizing cells can become. c. Cells needing greater surface area utilize membrane modifications such as folding, microvilli, etc. B. Microscopy Today (Science Focus Box) 1. Compound light microscopes use light rays focused by glass lenses. 2. Transmission electron microscopes (TEM) use electrons passing through specimen and focused by magnets. 3. Scanning electron microscopes (SEM) use electrons scanned across metal-coated specimen; secondary electrons given off by metal are collected by a detector. 4. Magnification is a function of wavelength; the shorter wavelengths of electrons allow greater magnification than the longer wavelengths of light rays. 5. Resolution is the minimum distance between two objects at which they can still be seen as separate objects. 6. Immunofluorescence microscopy uses fluorescent antibodies to reveal proteins in cells. 7. Confocal microscopy uses laser beam to focus on a shallow plane within the cell; this forms a series of optical sections from which a computer creates a three dimensional image. 8. Video-enhanced contrast microscopy accentuates the light and dark regions and may use a computer to contrast regions with false colors. 9. Bright-field, phase contrast, differential interference, and darkfield are different types of light microscopes. II Prokaryotic Cells 1. Prokaryotic cells lack a nucleus and are smaller and simpler than eukaryotic cells (which have a nucleus). 2. Prokaryotic cells are placed in two taxonomic domains: Bacteria and Archaea. Organisms in these two domains are structurally similar but biochemically different. A. The Structure of Bacteria 1. Bacteria are extremely small; average size is 1–1.5 μm wide and 2–6 μm long . 2. Bacteria occur in three basic shapes: spherical coccus, rod-shaped bacillus, and spiral spirillum (if rigid) or spirochete (if flexible). 3. Cell Envelope a. Includes the plasma membrane, the cell wall, and the glycocalyx. The plasma membrane is a lipid bilayer with imbedded and peripheral proteins; it regulates the movement of substances into and out of the cell. b. The plasma membrane can form internal pouches called mesosomes, which increase the internal surface area of the membrane for enzyme attachment. 26 c. d. The cell wall maintains the shape of the cell and is strengthened by peptidoglycan. The glycocalyx is a layer of polysaccharides on the outside of the cell wall; it is called a capsule if organized and not easily removed, or a slime layer if it is not well-organized and is easily removed. 4. Cytoplasm a. The cytoplasm is a semifluid solution containing water, inorganic and organic molecules, and enzymes. b. The nucleoid is a region that contains the single, circular DNA molecule. c. Plasmids are small accessory (extrachromosomal) rings of DNA; they are not part of the bacterial genetic material. d. Ribosomes are particles with two RNA- and protein-containing subunits that synthesize proteins. e. Inclusion bodies in the cytoplasm are granules of stored substances. f. Cyanobacteria (also called blue-green bacteria) are bacteria that photosynthesize; they lack chloroplasts but have thylakoids containing chlorophyll and other pigments. 5. Appendages a. Motile bacteria usually have flagella; the filament, hook, and basal body work to rotate the flagellum like a propeller to move through fluid medium. b. Fimbriae are small, bristlelike fibers that attach to an appropriate surface. c. Sex pili are tubes used by bacteria to pass DNA from cell to cell. B. The Structure of Archaea 1. In addition to spheres, rods, and spirals, Archaea can be lobed, platelike, or irregular. 2. The cell wall contains various polysaccharides and proteins rather than peptidoglycan. 3. The membrane lipids are composed of glycerol bonded to hydrocarbons, not fatty acids. 4. The DNA and RNA base sequences are closer to eukaryotes than bacteria. 5. Many Archaea are found in extremely salty or hot environments; they may have been the first type of cell to evolve. III Eukaryotic Cells 1. Eukaryotic cells are members of the domain Eukarya, which includes the protists, fungi, plants, and animals. 2. A membrane-bounded nucleus houses DNA; the nucleus may have originated as an invagination of the plasma membrane. 3. Eukaryotic cells are much larger than prokaryotic cells, and therefore have less surface area per volume. 4. Eukaryotic cells are compartmentalized; they contain small structures called organelles that perform specific functions. 5. Some eukaryotic cells (e.g., plant cells) have a cell wall containing cellulose; plasmodesmata are channels in a cell wall that allow cytoplasmic strands to extend between adjacent cells. A. The Structure of Eukaryotic Cells 1. The nucleus communicates with ribosomes in the cytoplasm. 2. The organelles of the endomembrane system communicate with one another; each organelle contains its own set of enzymes and produces its own products, which move from one organelle to another by transport vesicles. 3. The energy-related mitochondria (plant and animal cells) and chloroplasts (plant cells) do not communicate with other organelles; they contain their own DNA and are self-sufficient. 4. The cytoskeleton is a lattice of protein fibers that maintains the shape of the cell and assists in movement of the organelles. B. Cell Fractionation and Differential Centrifugation (Science Focus Box) 1. Cell fractionation allows the researcher to isolate and individually study the organelles of a cell. 2. Differential centrifugation separates the cellular components by size and density. 3. Using these two techniques, researchers can obtain pure preparations of any cell component. C. The Nucleus and Ribosomes a. The nucleus has a diameter of about 5 μm. b. Chromatin is a threadlike material that coils into chromosomes just before cell division occurs; contains DNA, protein, and some RNA. c. Nucleoplasm is the semifluid medium of the nucleus. 27 d. Chromosomes are rodlike structures formed during cell division; composed of coiled or folded chromatin. a. The nucleolus is a dark region of chromatin inside the nucleus; it is the site where ribosomal RNA (rRNA) joins with proteins to form ribosomes. b. The nucleus is separated from the cytoplasm by the nuclear envelope, which contains nuclear pores to permit passage of substances (e.g., ribosomal subunits, messenger RNA, proteins, etc.) in and out of the nucleus c. Ribosomes are the site of protein synthesis in the cell. In eukaryotic cells, ribosomes may occur freely or in groups called polyribosomes. d. Ribosomes receive messenger RNA (mRNA) from the nucleus, which instructs the ribosomes of the correct sequence of amino acids in a protein to be synthesized. D. The Endomembrane System 1. The endomembrane system is a series of intracellular membranes that compartmentalize the cell. 2. It consists of the nuclear envelope, the membranes of the endoplasmic reticulum, the Golgi apparatus, and several types of vesicles. 3. Endoplasmic Reticulum a. The endoplasmic reticulum (ER) is a system of membrane channels and saccules (flattened vesicles) continuous with the outer membrane of the nuclear envelope. b. Rough ER is studded with ribosomes on the cytoplasm side; it is the site where proteins are synthesized and enter the ER interior for processing and modification. c. Smooth ER is continuous with rough ER but lacks ribosomes; it is a site of various synthetic processes, detoxification, and storage; smooth ER forms transport vesicles. 4. The Golgi Apparatus a. It is named for Camillo Golgi, who discovered it in 1898. b. The Golgi apparatus consists of a stack of slightly curved saccules. c. The Golgi apparatus receives protein-filled vesicles that bud from the rough ER and lipid-filled vesicles from the smooth ER. d. Enzymes within the Golgi apparatus modify the carbohydrates that were placed on proteins in the ER; proteins and lipids are sorted and packaged. e. Vesicles formed from the membrane of the outer face of the Golgi apparatus move to different locations in a cell; at the plasma membrane they discharge their contents as secretions, a process called exocytosis because substances exit the cell. 5. Lysosomes a. Lysosomes are membrane-bounded vesicles produced by the Golgi apparatus. b. Lysosomes contain powerful digestive enzymes and are highly acidic. c. Macromolecules enter a cell by vesicle formation; lysosomes fuse with vesicles and digest the contents of the vesicle. d. White blood cells that engulf bacteria use lysosomes to digest the bacteria. e. Autodigestion occurs when lysosomes digest parts of cells. f. Lysosomes participate in apoptosis, or programmed cell death, a normal part of development. 6. Endomembrane System Summary a. Proteins produced in rough ER and lipids from smooth ER are carried in vesicles to the Golgi apparatus. b. The Golgi apparatus modifies these products and then sorts and packages them into vesicles that go to various cell destinations. c. Secretory vesicles carry products to the membrane where exocytosis produces secretions. d. Lysosomes fuse with incoming vesicles and digest macromolecules. E. Peroxisomes and Vacuoles 1. Peroxisomes are membrane-bounded vesicles that contain specific enzymes. a. Peroxisome action results in production of hydrogen peroxide. b. Hydrogen peroxide (H2O2) is broken down to water and oxygen by catalase. c. Peroxisomes in the liver produce bile salts from cholesterol and also break down fats. d. Peroxisomes also occur in germinating seeds where they convert oils into sugars used as nutrients by the growing plant. 2. Vacuoles a. Vacuoles are mebranous sacs and are larger than vesicles. 28 b. c. d. Contractile vacuoles in some protists rid the cell of excess water. Digestive vacuoles digest nutrients. Vacuoles generally store substances, e.g., plant vacuoles contain water, sugars, salts, pigments, and toxic molecules e. The central vacuole of a plant cell maintins turgor pressure within the cell, stores nutrients and wastes, and degrades organelles as the cell ages. F. Energy-Related Organelles 1. Chloroplasts are membranous organelles (a type of plastid) that serve as the site of photosynthesis. a. Photosynthesis is represented by the equation: solar energy + carbon dioxide + water → carbohydrate + oxygen b. Only plants, algae, and certain bacteria are capable of conducting photosynthesis. c. The chloroplast is bound by a double membrane organized into flattened disc-like sacs called thylakoids formed from a third membrane; a stack of thylakoids is a granum. d. Chlorophyll and other pigments capture solar energy, and the enzymes which synthesize carbohydrates are located in the chloroplasts. e. Chloroplasts have both their own DNA and ribosomes, supporting the endosymbiotic hypothesis. f. Other types of plastids, which differ in color, form, and function from chloroplasts, include chromoplasts and leucoplasts. 2. Mitochondria are surrounded by a double membrane: the inner membrane surrounds the matrix and is convoluted to form cristae. a. Mitochondria are smaller than chloroplasts, and often vary their shape. b. Mitochondria also can be fixed in one location or form long, moving chains. c. Mitochondria contain ribosomes and their own DNA. d. The matrix of the mitochondria is concentrated with enzymes that break down carbohydrates. e. ATP production occurs on the cristae. f. More than forty different diseases involving mitochondria have been described. G. The Cytoskeleton 1. The cytoskeleton is a network of connected filaments and tubules; it extends from the nucleus to the plasma membrane in eukaryotes. a. Electron microscopy reveals an organized cytosol. b. Immunofluorescence microscopy identifies protein fibers. c. Elements of the cytoskeleton include: actin filaments, intermediate filaments, and microtubules. 2. Actin Filaments a. Actin filaments are long, thin fibers (about 7 nm in diameter) that occur in bundles or meshlike networks. b. The actin filament consists of two chains of globular actin monomers twisted to form a helix. c. Actin filaments play a structural role, forming a dense complex web just under the plasma membrane; this accounts for the formation of pseudopods in amoeboid movement. d. Actin filaments in microvilli of intestinal cells likely shorten or extend cell into intestine. e. In plant cells, they form tracks along which chloroplasts circulate. 29 f. Actin filaments move by interacting with myosin; myosin combines with and splits ATP, binding to actin and changing configuration to pull actin filament forward. g. Similar action accounts for pinching off cells during cell division. 3. Intermediate Filaments a. Intermediate filaments are 8–11 nm in diameter, between actin filaments and microtubules in size. b. They are rope-like assemblies of fibrous polypeptides. c. Some support the nuclear envelope; others support plasma membrane and form cell-to-cell junctions. 4. Microtubules a. Microtubules are small hollow cylinders (25 nm in diameter and from 0.2–25 μm in length). b. Microtubules are composed of a globular protein tubulin that occurs as α tubulin and β tubulin. c. Assembly brings these two together as dimers and the dimers arrange themselves in rows. d. Regulation of microtubule assembly is under control of a microtubule organizing center (MTOC): the main MTOC is called a centrosome. e. Microtubules radiate from the MTOC, helping maintain the shape of cells and acting as tracks along which organelles move. f. Similar to actin-myosin, the motor molecules kinesin and dynein are associated with microtubules. g. Different kinds of kinesin proteins specialize to move one kind of vesicle or cell organelle. h. Cytoplasmic dynein is similar to the molecule dynein found in flagella. 5. Centrioles a. Centrioles are short cylinders with a ring pattern (9 + 0) of microtubule triplets. b. In animal cells and most protists, centrosome contains two centrioles lying at right angles to each other. c. Plant and fungal cells have the equivalent of a centrosome, but they do not contain centrioles. d. Centrioles serve as basal bodies for cilia and flagella. 6. Cilia and Flagella a. Cilia are short, usually numerous hairlike projections that can move in an undulating fashion (e.g., Paramecium; lining of human upper respiratory tract). b. Flagella are longer, usually fewer, projections that move in whip-like fashion (e.g., sperm cells). c. Both have similar construction, but differ from prokaryotic flagella. 1) Membrane-bounded cylinders enclose a matrix containing a cylinder of nine pairs of microtubules encircling two single microtubules (9 + 2 pattern of microtubules). 2) Cilia and flagella move when the microtubules slide past one another. 3) Cilia and flagella have a basal body at base with the same arrangement of microtubule triples as centrioles. 4) Cilia and flagella grow by the addition of tubulin dimers to their tips. Critical Thinking Question 1. When living tissues are viewed through the microscope, the cells reveal complex internal movements that amazed the early microscopists. “Vitalism” is the belief that some additional vital force is necessary to explain life and this movement inside cells. Why are modern biologists, who see these complex cells and their movements every day, not “vitalists?” Question 2. Freezing and thawing of vegetables destroys their structure because small sharp ice crystals pierce the cell walls and destroy the structure. Although meat lacks cell walls, repeated freezing and thawing produces the bad taste of “freezer burn.” What is the main organelle involved in this partial digestion of the cells in the meat? Question 3. How is cell biology linked to the development of scientific technology 30 CHAPTER 5 MEMBRANE STRUCTURE AND FUNCTION The complex structure and function of the plasma membrane are described, along with the macromolecules that comprise the membrane. The mechanisms by which substances move in and out of cells are discussed, as are the general chemical processes of diffusion and osmosis. Important cell surface modifications and their significance (e.g., in cellular junctions) are also detailed. Chapter Outline 5.1 Membrane Models 1. In the early 1900s, researchers noted that lipid-soluble molecules entered cells more rapidly than water-soluble molecules, suggesting lipids are component of plasma membrane. 2. Later, chemical analysis revealed that the membrane contained phospholipids. 3. Gorter and Grendel (1925) found that the amount of phospholipid extracted from a red blood cell was just enough to form one bilayer; they also suggested the nonpolar tails were directed inward and polar heads outward. 4. To account for the permeability of membrane to nonlipid substances, Danielli and Davson (1940s) proposed the “sandwich” model, with a phospholipid bilayer between layers of protein. 5. Robertson (1950s) proposed that proteins were embedded in an outer membrane and that all membranes in cells had similar compositions—the “unit membrane” model. 6. Additional research showed great diversity in membrane structure and function. A. Fluid-Mosaic Model 1. In 1972, Singer and Nicolson introduced the currently accepted fluid-mosaic model. a. The plasma membrane is a phospholipid bilayer, in which protein molecules are embedded. b. Embedded proteins are scattered throughout membrane in an irregular pattern; this varies among membranes. 5.2 Plasma Membrane Structure and Function 1. The plasma membrane is a phospholipid bilayer with embedded proteins. 2. Phospholipids have both hydrophilic and hydrophobic regions; nonpolar tails (hydrophobic) are directed inward, polar heads (hydrophilic) are directed outward to face both extracellular and intracellular fluid. 3. The proteins form a mosaic pattern on the membrane. 4. Cholesterol is a lipid found in animal plasma membranes; it stiffens and strengthens the membrane. 5. Glycolipids have a structure similar to phospholipids except the hydrophilic head is a variety of sugar; they are protective and assist in various functions. 6. Glycoproteins have an attached carbohydrate chain of sugar that projects externally. 7. The plasma membrane is asymmetrical; glycolipids and proteins occur only on outside and cytoskeletal filaments attach to proteins only on the inside surface. 31 A. B. C. 5.3 A. Carbohydrate Chains 1. In animal cells, the glycocalyx is a “sugar coat” of carbohydrate chains; it has several functions. 2. Cells are unique in that they have highly varied carbohydrate chains (a “fingerprint”). 3. The immune system recognizes foreign tissues that have inappropriate carbohydrate chains. 4. Carbohydrate chains are the basis for A, B, and O blood groups in humans. Fluidity of the Plasma Membrane 1. At body temperature, the phospholipid bilayer has the consistency of olive oil. 2. The greater the concentration of unsaturated fatty acid residues, the more fluid the bilayer. 3. In each monolayer, the hydrocarbon tails wiggle, and entire phospholipid molecules can move sideways. 4. Phospholipid molecules rarely “flip-flop” from one layer to the other. 5. Fluidity of the phospholipid bilayer allows cells to be pliable. 6. Some proteins are held in place by cytoskeletal filaments; most drift in the fluid bilayer. The Functions of the Proteins 1. Plasma membrane and organelle membranes have unique proteins; red blood cells (RBC) plasma membrane contains 50+ types of proteins. 2. Membrane proteins determine most of the membrane’s functions. 3. Channel proteins allow a particular molecule to cross membrane freely (e.g., Cl channels). 4. Carrier proteins selectively interact with a specific molecule so it can cross the plasma membrane (e.g., Na+-K+ pump). 5. Cell recognition proteins are glycoproteins that allow the body’s immune system to distinguish between foreign invaders and body cells. 6. Receptor proteins are shaped so a specific molecule (e.g., hormone) can bind to it. 7. Enzymatic proteins carry out specific metabolic reactions. Permeability of the Plasma Membrane 1. The plasma membrane is differentially (selectively) permeable; only certain molecules can pass through. a. Small non-charged lipid molecules (alcohol, oxygen) pass through the membrane freely. b. Small polar molecules (carbon dioxide, water) move “down” a concentration gradient, i.e., from high to low concentration. c. Ions and charged molecules cannot readily pass through the hydrophobic component of the bilayer and usually combine with carrier proteins. 2. Both passive and active mechanisms move molecules across membrane. a. Passive transport moves molecules across membrane without expenditure of energy; includes diffusion and facilitated transport. b. Active transport requires a carrier protein and uses energy (ATP) to move molecules across a plasma membrane; includes active transport, exocytosis, endocytosis, and pinocytosis. Diffusion and Osmosis 1. Diffusion is the movement of molecules from higher to lower concentration (i.e., “down” the concentration gradient). a. A solution contains a solute, usually a solid, and a solvent, usually a liquid. b. In the case of a dye diffusing in water, the dye is a solute and water is the solvent. c. Once a solute is evenly distributed, random movement continues but with no net change. d. Membrane chemical and physical properties allow only a few types of molecules to cross by diffusion. e. Gases readily diffuse through the lipid bilayer; e.g., the movement of oxygen from air sacs (alveoli) to the blood in lung capillaries depends on the concentration of oxygen in alveoli. f. Temperature, pressure, electrical currents, and molecular size influence the rate of diffusion. 2. Osmosis is the diffusion of water across a differentially (selectively) permeable membrane. a. Osmosis is illustrated by the thistle tube example: 1) A differentially permeable membrane separates two solutions. 32 2) The beaker has more water (lower percentage of solute) and the thistle tube has less water (higher percentage of solute). 3) The membrane does not permit passage of the solute; water enters but the solute does not exit. 4) The membrane permits passage of water with a net movement of water from the beaker to the inside of the thistle tube. b. Osmotic pressure is the pressure that develops in such a system due to osmosis. c. Osmotic pressure results in water being absorbed by the kidneys and water being taken up from tissue fluid. 3. Tonicity is strength of a solution with respect to osmotic pressure. a. Isotonic solutions occur where the relative solute concentrations of two solutions are equal; a 0.9% salt solution is used in injections because it is isotonic to red blood cells (RBCs). b. A hypotonic solution has a solute concentration that is less than another solution; when a cell is placed in a hypotonic solution, water enters the cell and it may undergo cytolysis (“cell bursting”). c. Swelling of a plant cell in a hypotonic solution creates turgor pressure; this is how plants maintain an erect position. d. A hypertonic solution has a solute concentration that is higher than another solution; when a cell is placed in a hypertonic solution, it shrivels (a condition called crenation). e. Plasmolysis is shrinking of the cytoplasm due to osmosis in a hypertonic solution; as the central vacuole loses water, the plasma membrane pulls away from the cell wall. B. Transport by Carrier Proteins 1. The plasma membrane impedes passage of most substances but many molecules enter or leave at rapid rates. 2. Carrier proteins are membrane proteins that combine with and transport only one type of molecule or ion; they are believed to undergo a change in shape to move the molecule across the membrane. 3. Facilitated transport is the transport of a specific solute “down” or “with” its concentration gradient (from high to low), facilitated by a carrier protein; glucose and amino acids move across the membrane in this way. 4. Active transport is transport of a specific solute across plasma membranes “up” or “against” (from low to high) its concentration gradient through use of cellular energy (ATP). a. Iodine is concentrated in cells of thyroid gland, glucose is completely absorbed into lining of digestive tract, and sodium is mostly reabsorbed by kidney tubule lining. b. Active transport requires both carrier proteins and ATP; therefore cells must have high number of mitochondria near membranes where active transport occurs. c. Proteins involved in active transport are often called “pumps”; the sodium-potassium pump is an important carrier system in nerve and muscle cells. d. Salt (NaCl) crosses a plasma membrane because sodium ions are pumped across, and the chloride ion is attracted to the sodium ion and simply diffuses across specific channels in the membrane. 5. Membrane-Assisted Transport a. In exocytosis, a vesicle formed by the Golgi apparatus fuses with the plasma membrane as secretion occurs; insulin leaves insulin-secreting cells by this method. b. During endocytosis, cells take in substances by vesicle formation as plasma membrane pinches off by either phagocytosis, pinocytosis, or receptor-mediated endocytosis. c. In phagocytosis, cells engulf large particles (e.g., bacteria), forming an endocytic vesicle. 1) Phagocytosis is commonly performed by ameboid-type cells (e.g., amoebas and macrophages). 2) When the endocytic vesicle fuses with a lysosome, digestion of the internalized substance occurs. d. Pinocytosis occurs when vesicles form around a liquid or very small particles; this is only visible with electron microscopy. e. Receptor-mediated endocytosis, a form of pinocytosis, occurs when specific macromolecules bind to plasma membrane receptors. 33 1) The receptor proteins are shaped to fit with specific substances (vitamin, hormone, lipoprotein molecule, etc.), and are found at one location in the plasma membrane. 2) This location is a coated pit with a layer of fibrous protein on the cytoplasmic side; when the vesicle is uncoated, it may fuse with a lysosome. 3) Pits are associated with exchange of substances between cells (e.g., maternal and fetal blood). 4) This system is selective and more efficient than pinocytosis; it is important in moving substances from maternal to fetal blood. 5) Cholesterol (transported in a molecule called a low-density lipoprotein, LDL) enters a cell from the bloodstream via receptors in coated pits; in familial hypocholesterolemia, the LDL receptor cannot bind to the coated pit and the excess cholesterol accumulates in the circulatory system. 5.4 Modification of Cell Surfaces A. Cell Surfaces in Animals 1. Junctions Between Cells are points of contact between cells that allow them to behave in a coordinated manner. a. Anchoring junctions mechanically attach adjacent cells. b. In adhesion junctions, internal cytoplasmic plaques, firmly attached to cytoskeleton within each cell are joined by intercellular filaments; they hold cells together where tissues stretch (e.g., in heart, stomach, bladder). c. In desmosomes, a single point of attachment between adjacent cells connects the cytoskeletons of adjacent cells. d. In tight junctions, plasma membrane proteins attach in zipper-like fastenings; they hold cells together so tightly that the tissues are barriers (e.g., epithelial lining of stomach, kidney tubules, blood-brain barrier). e. A gap junction allows cells to communicate; formed when two identical plasma membrane channels join. 1) They provide strength to the cells involved and allow the movement of small molecules and ions from the cytoplasm of one cell to the cytoplasm of the other cell. 2) Gap junctions permit flow of ions for heart muscle and smooth muscle cells to contract. 2. The extracellular matrix is a meshwork of polysaccharides and proteins produced by animal cells. a. Collagen gives the matrix strength and elastin gives it resilience. b. Fibronectins and laminins bind to membrane receptors and permit communication between matrix and cytoplasm; these proteins also form “highways” that direct the migration of cells during development. c. Proteoglycans are glycoproteins that provide a packing gel that joins the various proteins in matrix and most likely regulate signaling proteins that bind to receptors in the plasma protein. B. Plant Cell Walls 1. Plant cells are surrounded by a porous cell wall; it varies in thickness, depending on the function of the cell. 2. Plant cells have a primary cell wall composed of cellulose polymers united into threadlike microfibrils that form fibrils. 3. Cellulose fibrils form a framework whose spaces are filled by non-cellulose molecules. 4. Pectins allow the cell wall to stretch and are abundant in the middle lamella that holds cells together. 5. Non-cellulose polysaccharides harden the wall of mature cells. 6. Lignin adds strength and is a common ingredient of secondary cell walls in woody plants. 7. Plasmodesmata are narrow membrane-lined channels that pass through cell walls of neighboring cells and connect their cytoplasms, allowing direct exchange of molecules and ions between neighboring plant cells. 34 Critical Thinking Question 1. If you do not water your houseplants, they will first wilt, then eventually die. Why do wilting and dying not occur at the same time? Answer: A plant maintains its rigid shape by holding water inside a large central vacuole; the water pushes against the plasma membrane, which pushes against the rigid cell wall, and this turgor pressure holds the plant rigid. The plant wilts when the water level drops to where there is not enough turgor pressure to maintain rigidity, but there is plenty of water for life processes to continue. Loss of additional water will dehydrate the cell and eventually cause death of plant tissue. Question 2. You can “peel” a raw egg without breaking the membrane or “melt” away the shell in vinegar. If you place it in a glass of distilled, deionized water, it will swell in size until it breaks. Why is the flow one-way? Answer: The egg white is albumin, a huge protein molecule that cannot pass through the membrane. Water is a small molecule that can easily pass either way across the membrane. Since the water outside is 100% water and the albumin cannot leave, the percentage of water inside is always lower than 100 percent; thus the water molecules will continue to move from higher concentration outside the egg to a lower concentration inside. Question 3. The DNA of a cell codes for sequences of amino acids in proteins but the main component of a plasma membrane is phospholipids, a molecule that is not a protein. Where does the “new” plasma membrane come from when cells reproduce? Answer: As a lipid, the plasma membrane grows by adding lipid molecules from the cellular environment, a process called accretion. The unique proteins that are embedded in the membrane would be encoded by DNA but all non-protein elements of a cell must be developed by other cellular mechanisms. CHAPTER 6 METABOLISM: ENERGY AND ENZYMES The nature of energy and the laws of thermodynamics are discussed, followed by a detailed description of energy transformations that occur within the cell. The chemistry and functions of ATP are described. The role of enzymes in metabolism, oxidationreduction reactions, and the cellular organelles in which these reactions take place are detailed. Chapter Outline 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion; all moving objects have kinetic energy. 3. Potential energy is stored energy. 4. Food is chemical energy; it contains potential energy. 5. Chemical energy can be converted into mechanical energy, e.g., muscle movement. B. Two Laws of Thermodynamics 1. First law of thermodynamics (also called the law of conservation of energy) a. Energy cannot be created or destroyed, but it can be changed from one form to another. 35 b. C. 6.2 A. B. C. 6.3 In an ecosystem, solar energy is converted to chemical energy by the process of photosynthesis; some of the chemical energy in the plant is converted to chemical energy in an animal, which in turn can become mechanical energy or heat loss. c. Neither the plant nor the animal create energy, they convert it from one form to another. d. Likewise, energy is not destroyed; some becomes heat that dissipates into the environment. 2. Second law of thermodynamics a. Energy cannot be changed from one form into another without a loss of usable energy. b. Heat is a form of energy that dissipates into the environment; heat can never be converted back to another form of energy. Cells and Entropy 1. Every energy transformation makes the universe less organized and more disordered; entropy is the term used to indicate the relative amount of disorganization. 2. When ions distribute randomly across a membrane, entropy has increased. 3. Organized/usable forms of energy (as in the glucose molecule) have relatively low entropy; unorganized/less stable forms have relatively high entropy. 4. Energy conversions result in heat; therefore, the entropy of the universe is always increasing. 5. Living things depend on a constant supply of energy from the sun, because the ultimate fate of all solar energy in the biosphere is to become randomized in the universe as heat; the living cell is a temporary repository of order purchased at the cost of a constant flow of energy. Metabolic Reactions and Energy Transformations 1. Metabolism is the sum of all the biochemical reactions in a cell. 2. In the reaction A + B = C + D, A and B are reactants and C and D are products. 3. Free energy (G) is the amount of energy that is free to do work after a chemical reaction. 4. Change in free energy is noted as G; a negative G means that products have less free energy than reactants; the reaction occurs spontaneously. 5. Exergonic reactions have a negative G and energy is released. 6. Endergonic reactions have a positive G; products have more energy than reactants; such reactions can only occur with an input of energy. ATP: Energy for Cells 1. Adenosine triphosphate (ATP) is the energy currency of cells; when cells need energy, they “spend” ATP. 2. ATP is an energy carrier for many different types of reactions. 3. When ATP is converted into ADP + P, the energy released is sufficient for biological reactions with little wasted. 4. ATP breakdown is coupled to endergonic reactions in a way that minimizes energy loss. 5. ATP is a nucleotide composed of the base adenine and the 5-carbon sugar ribose and three phosphate groups. 6. When one phosphate group is removed, about 7.3 kcal of energy is released per mole. Coupled Reactions 1. A coupled reaction occurs when energy released by an exergonic reaction is used to drive an endergonic reaction. 2. ATP breakdown is often coupled to cellular reactions that require energy. 3. ATP supply is maintained by breakdown of glucose during cellular respiration. 4. Only 39% of the chemical energy of glucose is transformed into ATP; 61% is lost as heat. ATP can have any of three functions. 1. Chemical Work: ATP supplies energy to synthesize molecules that make up the cell. 2. Transport Work: ATP supplies energy to pump substances across the plasma membrane. 3. Mechanical Work: ATP supplies energy needed to perform mechanical processes (e.g., muscle contraction, propel cilia, etc.). Metabolic Pathways and Enzymes 1. A metabolic pathway is an orderly sequence of linked reactions; each step is catalyzed by a specific enzyme. 2. Metabolic pathways begin with a particular reactant, end with a particular end product(s), and may have many intermediate steps. 36 3. In many instances, one pathway leads to the next; since pathways often have one or more molecules in common, one pathway can lead to several others. 4. Metabolic energy is captured more easily if it is released in small increments. 5. A reactant is the substance that is converted into a product by the reaction; often many intermediate steps occur. 6. Each step in a series of chemical reactions requires a specific enzyme. 7. Enzymes are catalysts that speed chemical reactions without the enzyme being affected by the reaction. 8. Every enzyme is specific in its action and catalyzes only one reaction or one type of reaction. 9. A substrate is a reactant for an enzymatic reaction. A. Energy of Activation 1. Molecules often do not react with each other unless activated in some way. 2. For metabolic reactions to occur in a cell, an enzyme must usually be present. 3. The energy of activation (Ea) is the energy that must be added to cause molecules to react; without an enzyme (i.e., in a reaction vessel in the laboratory) this energy may be provided by heat, which causes an increase in the number of molecular collisions. B. Enzyme-Substrate Complex 1. Enzymes speed chemical reactions by lowering the energy of activation (E a) by forming a complex with their substrate(s) at the active site. a. An active site is a small region on the surface of the enzyme where the substrate(s) bind. b. When a substrate binds to an enzyme, the active site undergoes a slight change in shape that facilitates the reaction. This is called the induced fit model of enzyme catalysis. 2. Only a small amount of enzyme is needed in a cell because enzymes are not consumed during catalysis. 3. Some enzymes (e.g., trypsin) actually participate in the reaction. 4. A particular reactant(s) may produce more than one type of product(s). a. Presence or absence of enzyme determines which reaction takes place. b. If reactants can form more than one product, the enzymes present determine which product is formed. 5. Every cell reaction requires its specific enzyme; enzymes are sometimes named for substrates by adding “-ase.” C. Factors Affecting Enzymatic Speed 1. Substrate concentration. a. Because molecules must collide to react, enzyme activity increases as substrate concentration increases; as more substrate molecules fill active sites, more product is produced per unit time. 2. Temperature and pH a. As temperature rises, enzyme activity increases because there are more enzyme-substrate collisions. b. Enzyme activity declines rapidly when enzyme is denatured at a certain temperature, due to a change in shape of the enzyme. c. Every enzyme has optimal pH at which its rate of reaction is optimal. d. A change in pH can alter the ionization of the R groups of the amino acids in the enzyme, thereby disrupting the enzyme’s activity. 3. Enzyme concentration a. The amount of active enzyme can regulate the rate of an enzymatic reaction. b. Cells can activate specific genes when certain enzymes are needed. c. Enzyme Cofactors 1) Many enzymes require an inorganic ion or non-protein cofactor to function. 2) Inorganic cofactors are ions of metals. 3) A coenzyme is an organic cofactor, which assists the enzyme (i.e., it may actually contribute atoms to the reaction). 4) Vitamins are small organic molecules required in trace amounts for synthesis of coenzymes; they become part of a coenzyme’s molecular structure; vitamin deficiency causes a lack of a specific coenzyme and therefore a lack of its enzymatic action. 37 5) Phosphorylation of enzymes occurs when signal proteins turn on kinases, which then activate specific enzymes; some hormones use this mechanism. d. Enzyme inhibition occurs when a substance (called an inhibitor) binds to an enzyme and decreases its activity; normally, enzyme inhibition is reversible. 1) In competitive inhibition, the substrate and the inhibitor are both able to bind to the enzyme’s active site. 2) In noncompetitive inhibition, the inhibitor binds to the enzyme at a location other than the active site (the allosteric site), changing the shape of the enzyme and rendering it unable to bind to its substrate. 3) Competitive and noncompetitive inhibition are both examples of feedback inhibition. 4) In irreversible inhibition, the inhibitor permanently inactivates or destroys the enzyme; cyanide, mercury, and lead are irreversible inhibitors for several specific enzymes. 6.4 Oxidation-Reduction and the Flow of Energy 1. 2. 3. 4. A. B. C. D. In oxidation-reduction (redox) reactions, electrons pass from one molecule to another. Oxidation is the loss of electrons. Reduction is the gain of electrons. Both reactions occur at the same time because one molecule accepts electrons given up by another molecule. Photosynthesis 1. Photosynthesis uses energy to combine carbon dioxide and water to produce glucose in the formula: 6 CO2 + 6 H2O + energy = C6H12O6 + 6 O2 2. When hydrogen atoms are transferred to carbon dioxide from water, water has been oxidized and carbon dioxide has been reduced. 3. Input of energy is needed to produce the high-energy glucose molecule. 4. Chloroplasts capture solar energy and convert it by way of an electron transport system into the chemical energy of ATP. 5. ATP is used along with hydrogen atoms to reduce glucose; when NADP+ (nicotinamide adenine dinucleotide phosphate) donates hydrogen atoms (H+ + e) to a substrate during photosynthesis, the substrate has accepted electrons and is therefore reduced. 6. The reaction that reduces NADP+ is: NADP+ + 2e + H+ = NADPH Cellular Respiration 1. The overall equation for cellular respiration is opposite that of photosynthesis: C6H12O6 + 6 O2 = 6 CO2 + 6 H2O + energy 2. When NAD removes hydrogen atoms (H+ + e-) during cellular respiration, the substrate has lost electrons and is therefore oxidized. 3. At the end of cellular respiration, glucose has been oxidized to carbon dioxide and water and ATP molecules have been produced. 4. In metabolic pathways, most oxidations involve the coenzyme NAD+ (nicotinamide adenine dinucleotide); the molecule accepts two electrons but only one hydrogen ion: NAD + + 2e + H+ = NADH Electron Transport Chain 1. Both photosynthesis and respiration use an electron transport chain consisting of membrane-bound carriers that pass electrons from one carrier to another. 2. High-energy electrons are delivered to the system and low-energy electrons leave it. 3. The overall effect is a series of redox reactions; every time electrons transfer to a new carrier, energy is released for the production of ATP. ATP Production 1. ATP synthesis is coupled to the electron transport system. 2. Peter Mitchell received the 1978 Nobel Prize for his chemiosmotic theory of ATP production. 3. In both mitochondria and chloroplasts, carriers of electron transport systems are located within a membrane. 38 4. 5. 6. 7. 8. H+ ions (protons) collect on one side of the membrane because they are pumped there by specific proteins. The electrochemical gradient thus established across the membrane is used to provide energy for ATP production. Enzymes and their carrier proteins, called ATP synthase complexes, span the membrane; each complex contains a channel that allows H + ions to flow down their electrochemical gradient. In photosynthesis, energized electrons lead to the pumping of hydrogen ions across the thylakoid membrane; as hydrogen ions flow through the ATP synthase complex, ATP is formed. During cellular respiration, glucose breakdown provides energy for a hydrogen ion gradient on the inner membrane of the mitochondria that also couples hydrogen ion flow with ATP formation. Critical Thinking Question 1. Why does the growth of a seven pound baby into a 100 pound adolescent not violate the second law of thermodynamics? Answer: To produce and support the extra 93 pounds of living tissue, a much larger amount, well over 1,000 pounds of food had to be digested. Thus, we are “islands of complexity” built up amid a sea of entropy. Such islands of organized life can continue to be built up as long as there is an outside source of energy--the sun, providing that input of energy. In physics, this would be described as an “open system” since energy is continually supplied from the outside (the sun) to keep the systems on earth “running.” Question 2. What are the key organelles that allow energy to flow through living systems? Answer: The chloroplast and mitochondrion temporarily store energy as chemical energy so some of it flows through living systems. When chloroplasts carry on photosynthesis, solar energy is used to produce carbohydrates, and when mitochondria carry on cellular respiration, the energy stored in carbohydrates is converted to energy stored in ATP. All organisms make use of energy stored in ATP before the energy is eventually lost as heat to the universe. 39 CHAPTER 7 PHOTOSYNTHESIS Photosynthesis is the biochemical process by which organic molecules (i.e., sugars) are synthesized for use by organisms throughout the food web. Photosynthesis takes place in the chloroplasts of plant cells (and certain other types of organisms). The process includes the “light reactions,” in which solar energy is captured, and the “Calvin Cycle reactions” (light independent reactions, “dark reactions”), in which carbohydrates are synthesized. The two pathways of light reactions are described, as are the Calvin Cycle reactions. The pigments involved in photosynthesis are discussed, as are the C3, C4, and CAM photosynthetic mechanisms. Chapter Outline 7.1 Photosynthetic Organisms 1. Photosynthetic organisms (algae, plants, and cyanobacteria) transform solar energy into carbohydrates. 2. Photosynthetic organisms (plants, algae, cyanobacteria) are called autotrophs because they produce their own food. 3. Organisms that must take in preformed organic molecules are called heterotrophs. 4. Both autotrophs and heterotrophs use organic molecules produced by photosynthesis as chemical building blocks and as a source of energy. A. Flowering Plants as Photosynthesizers 1. Raw materials for photosynthesis are carbon dioxide and water. 2. Roots absorb water that moves up vascular tissue in the stem until it reaches the leaf veins. 3. Carbon dioxide enters a leaf through small openings called stomata. 4. Carbon dioxide and water diffuse into the chloroplasts, the organelles that carry on photosynthesis. 5. In chloroplasts, a double membrane encloses a fluid-filled space called the stroma. 6. An internal membrane system within the stroma forms flattened sacs called thylakoids, which in some cases are organized into stacks to form grana. 7. Spaces within all thylakoids are connected to form an inner compartment, the thylakoid space. 8. Chlorophyll and other pigments involved in absorption of solar energy reside within thylakoid membranes; these pigments absorb solar energy, and energize electrons prior to reduction of CO2 to a carbohydrate. 7.2 Plants as Solar Energy Converters 1. Only 42% of the solar radiation that hits the Earth’s atmosphere reaches surface; most is visible light. 2. Higher energy wavelengths are screened out by the ozone layer in the upper atmosphere. 3. Lower energy wavelengths are screened out by water vapor and CO 2. 4. Both the organic molecules within organisms and certain processes (e.g., vision, photosynthesis) are adapted to visible light, the radiation that is most prevalent in the environment. A. Photosynthetic Pigments 1. Photosynthetic pigments use primarily the visible light portion of the electromagnetic spectrum. 2. Pigments found in chlorophyll absorb various portions of visible light; this is called their absorption spectrum. 3. Two major photosynthetic pigments are chlorophyll a and chlorophyll b. 4. Both chlorophylls absorb violet, blue, and red wavelengths best. 5. Very little green light is absorbed; most is reflected (this is why leaves appear green). 6. Carotenoids are yellow-orange pigments that absorb light in violet, blue, and green regions. 40 7. When chlorophyll breaks down in the fall, the yellow-orange pigments in leaves show through. 8. Absorption and action spectrum a. A spectrophotometer measures the amount of light that passes through a sample. 1) As light is shone on a sample, some wavelengths are absorbed and others pass through the sample. 2) A graph of percent of light absorbed at each wavelength is a compound’s absorption spectrum. b. Action spectrum 1) Photosynthesis produces oxygen; the production rate of oxygen is used to measure the rate of photosynthesis. 2) Oxygen production and therefore photosynthetic activity is measured for plants under each specific wavelength; when plotted on a graph, this gives an action spectrum for a compound. 3) The action spectrum for chlorophyll resembles its absorption spectrum, thus indicating that chlorophyll contributes to photosynthesis. B. Photosynthetic Reaction 1. In 1930, van Niel showed that O2 given off by photosynthesis comes from water and not from CO2. 2. The net equation of photosynthesis reads: 6CO2 + 6H2O = C6 H12O6 + 6O2. C. Two Sets of Reactions 1. 2. 3. In 1905, Blackman proposed two sets of reactions for photosynthesis. Light reactions take place only in the presence of light. a. Light reactions are the energy-capturing reactions. b. Chlorophyl within thylakoid membranes absorbs solar energy and energizes electrons. c. When energized electrons move down an electron transport chain, energy is captured and used for ATP production. d. Energized electrons are also taken up by NADP +, converting it to NADPH. Calvin cycle reactions a. These reactions take place in the stroma; the reactions can occur in either the presence or the absence of light. b. These are synthetic reactions that use NADPH and ATP to reduce CO2. 7.3 Light Reactions 1. 2. 3. Two electron pathways operate in the thylakoid membrane: the noncyclic pathway and the cyclic pathway. Both pathways produce ATP; only the noncyclic pathway also produces NADPH. ATP production during photosynthesis is called photophosphorylation; therefore these pathways are also known as cyclic and noncyclic photophosphorylation. A. Noncyclic Electron Pathway 1. 2. 3. 4. 5. 6. 7. 8. This pathway occurs in the thylakoid membranes and requires participation of two light-gathering units: photosystem I (PS I) and photosystem II (PS II). A photosystem is a photosynthetic unit comprised of a pigment complex and an electron acceptor; solar energy is absorbed and high-energy electrons are generated. Each photosystem has a pigment complex of chlorophyll a, chlorophyll b, carotenoid, and electron acceptor molecules. Absorbed energy is passed from one pigment molecule to another until concentrated in reaction- center chlorophyll a molecules. Electrons in reaction-center chlorophyll a become excited, and escape to the electron-acceptor molecule. The noncyclic pathway begins with PSII; electrons move from H 2O through PS II to PS I and then on to NADP+. The PS II pigment complex absorbs solar energy; high-energy electrons (e) leave the reaction-center chlorophyll a molecule. PS II takes replacement electrons from H2O, which splits, releasing O2 and H+ ions: 41 H2O =2 H+ + 2 e + ½ O2. 9. Oxygen is released as oxygen gas (O2). 10. The H+ ions temporarily stay within the thylakoid space and contribute to a H + ion gradient. 11. As H+ flow down electrochemical gradient through ATP synthase complexes, chemiosmosis occurs. 12. Low-energy electrons leaving the electron transport system enter PS I. 13. When the PS I pigment complex absorbs solar energy, high-energy electrons leave reaction-center chlorophyll a and are captured by an electron acceptor. 14. The electron acceptor passes them on to NADP +. 15. NADP+ takes on an H+ to become NADPH: NADP+ + 2 e + H+ = NADPH. 16. NADPH and ATP (produced by noncyclic-flow electrons in the thylakoid membrane) are used by enzymes in the stroma during the light-independent (dark) reactions. B. Cyclic Electron Pathway 1. The cyclic electron pathway begins when the PS I antenna complex absorbs solar energy. 2. High-energy electrons leave PS I reaction-center chlorophyll a molecule. 3. Before they return, the electrons enter and travel down an electron transport chain. a. Electrons pass from a higher to a lower energy level. b. Energy released is stored in the form of a hydrogen (H +) gradient. c. When hydrogen ions flow down their electrochemical gradient through ATP synthase complexes, ATP production occurs. d. The electrons return to PSI rather than move on to NADP +--this is why it is called cyclic and also why no NADPH is produced. 4. It is possible that in plants, the cyclic flow of electrons is utilized only when CO 2 is in such limited supply that carbohydrate is not being produced. C. The Organization of the Thylakoid Membrane 1. PS II consists of a pigment complex and electron-acceptor molecules; it oxidizes H2O and produces O2. 2. The electron transport system consists of cytochrome complexes and transports electrons and pumps H+ ions into the thylakoid space. 3. PS I has a pigment complex and electron-acceptor molecules; it is associated with an enzyme that reduces NADP+ to NADPH. 4. ATP synthase complex has an H+ channel and ATP synthase; it produces ATP. D. ATP Production 1. The thylakoid space acts as a reservoir for H+ ions; each time H2O is split, two H+ remain. 2. Electrons move carrier-to-carrier, giving up energy used to pump H + from the stroma into the thylakoid space. 3. Flow of H+ from high to low concentration across thylakoid membrane provides energy to produce ATP from ADP + P by using an ATP synthase enzyme. 4. This is called chemiosmosis because ATP production is tied to an electrochemical (H+) gradient. 7.4 Calvin Cycle Reactions 1. The Calvin cycle is a series of reactions producing carbohydrates; these reactions follow the light reactions. 2. The cycle is named for Melvin Calvin who used a radioactive isotope of carbon to trace the reactions. 3. The Calvin cycle includes carbon dioxide fixation, carbon dioxide reduction, and regeneration of ribulose 1,5-bisphosphate (RuBP). A. Fixation of Carbon Dioxide 1. CO2 fixation is the attachment of CO2 to an organic compound called RuBP. 2. RuBP (ribulose bisphosphate) is a five-carbon molecule that combines with carbon dioxide; the resulting 6-carbon molecule then splits into two 3-carbon molecules. 3. The enzyme RuBP carboxylase (rubisco) speeds this reaction; this enzyme comprises 20–50% of the protein content of chloroplasts--it is an unusually slow enzyme. B. Reduction of Carbon Dioxide 42 1. 2. With the reduction of carbon dioxide, a 3PG (3-phosphoglycerate) molecule forms. Each of two 3PG molecules undergoes reduction to G3P (glyceraldehyde-3-phosphate) in two steps. 3. Light-dependent reactions provide NADPH (electrons) and ATP (energy) to reduce 3PG to G3P. C. Regeneration of RuBP 1. For every three turns of the Calvin cycle, five molecules of G3P are used to re-form three molecules of RuBP. 2. This reaction also uses ATP produced by the light reactions. D. The Importance of the Calvin Cycle 1. G3P, the product of the Calvin Cycle can be converted into many other molecules. 2. Glucose phosphate is one result of G3P metabolism; it is a common energy molecule. 3. Glucose phosphate can bond with fructose to form sucrose. 4. Glucose phosphate is the starting point for synthesis of starch and cellulose. 5. The hydrocarbon skeleton of G3P is used to form fatty acids and glycerol; the addition of nitrogen forms various amino acids. 7.5 Other Types of Photosynthesis 1. In C3 plants, the Calvin cycle fixes CO2 directly; the first molecule following CO2 fixation is 3PG. 2. In hot weather, stomata close to save water; CO2 concentration decreases in leaves; O2 increases. 3. This is called photorespiration since oxygen is taken up and CO2 is produced; this produces only one 3PG. A. C4 Photosynthesis 1. In a C3 plant, mesophyll cells contain well-formed chloroplasts, arranged in parallel layers. 2. In C4 plants, bundle sheath cells as well as the mesophyll cells contain chloroplasts. 3. In C4 leaf, mesophyll cells are arranged concentrically around the bundle sheath cells. 4. C3 plants use RuBP carboxylase to fix CO2 to RuBP in mesophyll; the first detected molecule is G3P. 5. C4 plants use the enzyme PEP carboxylase (PEPCase) to fix CO 2 to PEP (phosphoenolpyruvate, a C3 molecule); the end product is oxaloacetate (a C 4 molecule). 6. In C4 plants, CO2 is taken up in mesophyll cells and malate, a reduced form of oxaloacetate, is pumped into the bundle-sheath cells; here CO2 enters Calvin cycle. 7. In hot, dry climates, net photosynthetic rate of C4 plants (e.g., corn) is 2–3 times that of C4 plants. 8. Photorespiration does not occur in C4 leaves because PEPCase does not combine with O2; even when stomates are closed, CO2 is delivered to the Calvin cycle in bundle sheath cells. 9. C4 plants have advantage over C3 plants in hot and dry weather because photorespiration does not occur; e.g., bluegrass (C3) dominates lawns in early summer, whereas crabgrass (C4) takes over in the hot midsummer. B. CAM Photosynthesis 1. CAM (crassulacean-acid metabolism) plants form a C4 molecule at night when stomates can open without loss of water; found in many succulent desert plants including the family Crassulaceae. 2. At night, CAM plants use PEPCase to fix CO2 by forming C4 molecule stored in large vacuoles in mesophyll. 3. C4 formed at night is broken down to CO2 during the day and enters the Calvin cycle within the same cell, which now has NADPH and ATP available to it from the light-dependent reactions. 4. CAM plants open stomates only at night, allowing CO2 to enter photosynthesizing tissues; during the day, stomates are closed to conserve water but CO2 cannot enter photosynthesizing tissues. 5. Photosynthesis in a CAM plant is minimal, due to limited amount of CO 2 fixed at night; but this does allow CAM plants to live under stressful conditions. C. Photosynthesis and Adaptation to the Environment 1. Each method of photosynthesis has its advantages, depending on the environment. 43 2. 3. 4. C4 plants are adapted to areas of high light intensities, high temperatures, and limited rainfall. C3 plants do better in cooler climates. CAM plants do well in an arid environment. Critical Thinking Question 1. An astronaut is on a spaceship moving closer to the sun. The food supply is supplemented by an onboard greenhouse. The light intensity is increasing and for visual comfort, the astronaut must block out some sunlight. What color of glass-reflecting pane should be used to preserve the maximum photosynthesis, and why? Answer: Given no other limitations, blocking out green light would do the least damage to the plants since they use less light in the green wavelength and reflect green themselves. Theoretically, if all green light was prevented from entering, and the leaves absorbed all other visible wavelengths, the leaves in the space greenhouse would appear black! Question 2. The Dutchman Van Helmont grew a tree in a large pot and found the soil did not add to the weight of the tree. Unaware of the gases in the air, he therefore concluded a tree was made of water, the only thing else that he was aware was added in his experiment. How could you prove to Van Helmont that something in the air was also involved in making up the tree's substance? Answer: Many strategies are possible. 1) The air openings (stomata) on tree leaves are on the underside. Coating the upper surface of a leaf with petroleum jelly does not affect its growth; coating its underside does. 2) Sealing a plant inside a vacuum will kill it; however, early researchers contended a vital force had been shut off–the same force that kept a flame “alive.” This can be countered by sealing a plant inside a jar with only carbon dioxide; this will often improve growth. 3) Modern radioisotope tracers can be used to trace the flow of molecules through organisms. Students can invent other experimental procedures based upon plausible concepts. 44 CHAPTER 8 CELLULAR RESPIRATION This chapter undertakes a detailed study of the cellular process of respiration. The biochemical reactions occurring in the cytoplasm (glycolysis, fermentation) and in the mitochondria (citric acid cycle, electron transport, oxidative phosphorylation) are described, as are the molecules that participate in the reactions. Oxidation-reduction chemistry is emphasized. The energy yield (i.e., ATP) production is calculated for the complete oxidation of a glucose molecule, and is compared with the energy yield under anaerobic conditions. Other anabolic and catabolic reactions occurring in the cell are discussed. Chapter Outline 8.1 Cellular Respiration 1. Cellular respiration involves various metabolic pathways that break down carbohydrates and other metabolites with the concomitant buildup of ATP. 2. Cellular respiration consumes oxygen and produces CO2; because oxygen is required, cellular respiration is aerobic. 3. Cellular respiration usually involves the complete breakdown of glucose into CO 2 and H2O. 4. The net equation for glucose breakdown is: C6H12O6 + 6 O2 = 6 CO2 + 6 H2O + energy 5. Glucose is a high-energy molecule; CO2 and H2O are low-energy molecules; cellular respiration is thus exergonic because it releases energy. 6. Electrons are removed from substrates and received by oxygen, which combines with H + to become water. 7. Glucose is oxidized and O2 is reduced. 8. The buildup of ATP is an endergonic reaction (i.e., requires energy). 9. The reactions of cellular respiration allow energy in glucose to be released slowly; therefore ATP is produced gradually. 10. In contrast, if glucose were broken down rapidly, most of its energy would be lost as nonusable heat. 11. The breakdown of glucose yields synthesis of 36 or 38 ATP (depending on certain conditions); this preserves about 39% of the energy available in glucose. 12. This is relatively efficient compared to, for example, the 25% efficiency of a car burning gasoline. A. NAD+ and FAD 1. Each metabolic reaction in cellular respiration is catalyzed by a specific enzyme. 2. As a metabolite is oxidized, NAD+ (nicotinamide adenine dinucleotide) accepts two electrons and a hydrogen ion (H+); this results in NADH + H+. 3. Electrons received by NAD+ and FAD are high-energy electrons and are usually carried to the electron transport chain. 4. NAD+ is a coenzyme of oxidation-reduction since it both accepts and gives up electrons; thus, NAD+ is sometimes called a redox coenzyme 5. Only a small amount of NAD+ is needed in cells because each NAD+ molecule is used repeatedly. 6. FAD coenzyme of oxidation-reduction can replace NAD+; FAD accepts two electrons and two hydrogen ions to become FADH2. B. Phases of Cellular Respiration 1. Cellular respiration includes four phases: a. Glycolysis is the breakdown of glucose in the cytoplasm into two molecules of pyruvate. 1) Enough energy is released for an immediate yield of two ATP. 45 2. 2) Glycolysis takes place outside the mitochondria and does not utilize oxygen; it is therefore an anaerobic process. b. In the preparatory (prep) reaction, pyruvate enters a mitochondrion and is oxidized to a two-carbon acetyl group and CO2 is removed; this reaction occurs twice per glucose molecule. c. The citric acid cycle: 1) occurs in the matrix of the mitochondrion and produces NADH and FADH 2; 2) is a series of reactions that gives off CO2 and produces one ATP; 3) turns twice because two acetyl-CoA molecules enter the cycle per glucose molecule; 4) produces two immediate ATP molecules per glucose molecule. d. The electron transport chain: 1) is a series of carriers in the inner mitochondrial membrane that accept electrons from glucose--electrons are passed from carrier to carrier until received by oxygen; 2) passes electrons from higher to lower energy states, allowing energy to be released and stored for ATP production; 3) accounts for 32 or 34 ATP, depending on certain cell conditions. Pyruvate is a pivotal metabolite in cellular respiration. a. If O2 is not available to the cell, fermentation, an anaerobic process, occurs in the cytoplasm. b. During fermentation, glucose is incompletely metabolized to lactate, or to CO 2 and alcohol (depending on the organism). c. Fermentation results in a net gain of only two ATP per glucose molecule. 8.2 Outside the Mitochondria: Glycolysis 1. 2. 3. Glycolysis occurs in the cytoplasm outside the mitochondria. Glycolysis is the breakdown of glucose into two pyruvate molecules. Glycolysis is universally found in organisms; therefore, it likely evolved before the citric acid cycle and electron transport chain. A. Energy-Investment Steps 1. Glycolysis begins with the activation of glucose with two ATP; the glucose splits into two C 3 molecules known as G3P, each of which carries a phosphate group. B. Energy-Harvesting Steps 1. Oxidation of G3P occurs by removal of electrons and hydrogen ions. 2. Two electrons and one hydrogen ion are accepted by NAD +, resulting in two NADH; later, when the NADH molecules pass two electrons to another electron carrier, they become NAD+ again. 3. The oxidation of G3P and subsequent substrates results in four high-energy phosphate groups, which are used to synthesize four ATP molecules; this process is called substrate-level phosphorylation. 4. Two of four ATP molecules produced are required to replace two ATP molecules used in the initial phosphorylation of glucose; therefore there is a net gain of two ATP from glycolysis. 5. Pyruvate enters a mitochondrion (if oxygen is available) and cellular respiration ensues. 6. If oxygen is not available, fermentation occurs and pyruvate undergoes reduction. 8.3 Inside the Mitochondria 1. 2. 3. 4. 5. 6. 7. The next reactions of cellular respiration involve the preparatory reaction, the citric acid cycle, and the electron transport chain. In these reactions, the pyruvate from glycolysis is broken down completely to CO 2 and H2O. CO2 and ATP are transported out of the mitochondria into the cytoplasm. The H2O can remain in the mitochondria or within the cell, or it can enter the blood and be excreted by the kidneys. A mitochondrion has a double membrane with an intermembrane space (between the outer and inner membrane). Cristae are the inner folds of membrane that jut into the matrix, the innermost compartment of a mitochondrion that is filled with a gel-like fluid. The prep reaction and citric acid cycle enzymes are in the matrix; the electron transport chain is in the cristae. 46 8. Most of the ATP produced in cellular respiration is produced in the mitochondria; therefore, mitochondria are often called the “powerhouses” of the cell. A. Preparatory Reaction 1. The preparatory reaction connects glycolysis to the citric acid cycle. 2. In this reaction, pyruvate is converted to a two-carbon acetyl group, and is attached to coenzyme A, resulting in the compound acetyl-CoA. 3. This redox reaction removes electrons from pyruvate by a dehydrogenase enzyme, using NAD+ as a coenzyme. 4. This reaction occurs twice for each glucose molecule. 47 B. Citric Acid Cycle 1. The citric acid cycle occurs in the matrix of mitochondria. 2. The cycle is sometimes called the Krebs cycle, named for Sir Hans Krebs, who described the fundamentals of the reactions in the 1930s. 3. The cycle begins by the addition of a two-carbon acetyl group to a four-carbon molecule, forming a six-carbon citrate (citric acid) molecule. 4. In the subsequent reactions, at three different times two electrons and one hydrogen ion are accepted by NAD+, forming NADH. 5. At one time, two electrons and one hydrogen ion are accepted by FAD, forming FADH 2. 6. NADH and FADH2 carry these electrons to the electron transport chain. 7. Some energy is released and is used to synthesize ATP by substrate-level phosphorylation. 8. One high-energy metabolite accepts a phosphate group and transfers it to convert ADP to ATP. 9. The citric acid cycle turns twice for each original glucose molecule. 10. The products of the citric acid cycle (per glucose molecule) are 4 CO2, 2 ATP, 6 NADH and 2 FADH2. 11. The six carbon atoms in the glucose molecule have now become the carbon atoms of six CO 2 molecules, two from the prep reaction and four from the citric acid cycle. C. The Electron Transport Chain 1. The electron transport chain is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another. 2. Some of the protein carriers are cytochrome molecules, complex carbon rings with a heme (iron) group in the center. 3. NADH and FADH2 carry the electrons to the electron transport system.. 4. NADH gives up its electrons and becomes NAD +; the next carrier then gains electrons and is thereby reduced. 5. At each sequential redox reaction, energy is released to form ATP molecules. 6. Because O2 must be present for the proteins to work, this process is also called oxidative phosphorylation. 7. Oxygen serves as the terminal electron acceptor and combines with hydrogen ions to form water. 8. By the time electrons are received by O2, three ATP have been made. 9. When FADH2 delivers electrons to the electron transport system, two ATP are formed by the time the electrons are received by O2. 10. Coenzymes and ATP undergo recycling. a. Cell needs a limited supply of coenzymes NAD+ and FAD because they constantly recycle. b. Once NADH delivers electrons to the electron transport chain, it can accept more hydrogen atoms. c. ADP and phosphate also recycle. d. Efficiency of recycling NAD+, FAD, and ADP eliminates the need to continuously synthesize them anew. D. The Cristae of a Mitochondrion 1. The electron transport chain consists of three protein complexes and two protein mobile carriers that transport electrons. 2. The three protein complexes include NADH-Q reductase complex, the cytochrome reductase complex, and the cytochrome oxidase complex; the two protein mobile carriers are coenzyme Q and cytochrome c. 3. Energy released from the flow of electrons down the electron transport chain is used to pump H+ ions, which are carried by NADH and FADH2, into intermembrane space. 4. Accumulation of H+ ions in this intermembrane space creates a strong electrochemical gradient. 5. ATP synthase complexes are channel proteins that serve as enzymes for ATP synthesis. 6. As H+ ions flow from high to low concentration, ATP synthase synthesizes ATP by the reaction: ADP + P = ATP. 48 Chemiosmosis is the term used for ATP production tied to an electrochemical (H +) gradient across a membrane. 8. Respiratory poisons confirm the chemiosmotic nature of ATP synthesis (i.e., a poison that inhibits ATP synthesis increases the H+ gradient). 9. Once formed, ATP molecules diffuse out of the mitochondrial matrix through channel proteins. 10. ATP is the energy currency for all living things; all organisms must continuously produce high levels of ATP to survive. Energy Yield From Glucose Metabolism 1. Substrate-Level Phosphorylation a. Per glucose molecule, there is a net gain of two ATP from glycolysis in cytoplasm. b. The citric acid cycle in the matrix of the mitochondria produces two ATP per glucose. c. Thus, a total of four ATP are formed by substrate-level phosphorylation outside of the electron transport chain. 2. Electron Transport Chain and Chemiosmosis a. Most of the ATP is produced by the electron transport chain and chemiosmosis. b. Per glucose, ten NADH and two FADH2 molecules provide electrons and H+ ions to the electron transport chain. c. For each NADH formed within the mitochondrion, three ATP are produced. d. For each FADH2 formed by the citric acid cycle, two ATP are produced. e. For each NADH formed outside mitochondria by glycolysis, two ATP are produced as electrons are shuttled across the mitochondrial membrane by an organic molecule and delivered to FAD. 3. Efficiency of Cellular Respiration a. The energy difference between total reactants (glucose and O2) and products (CO2 and H2O) is 686 kcal. b. An ATP phosphate bond has an energy of 7.3 kcal; 36 to 38 ATP are produced during glucose breakdown for a total of at least 263 kcal. c. This efficiency is 263/686, or 39% of the available energy in glucose is transferred to ATP; the rest of the energy is lost as heat. Fermentation 1. Fermentation is an anaerobic (i.e., occurs in the absence of oxygen) process which consists of glycolysis plus reduction of pyruvate to either lactate or to alcohol and CO2 (depending on the organism). 2. NADH passes its electrons to pyruvate instead of to an electron transport chain; NAD + is then free to return and pick up more electrons during earlier reactions of glycolysis. 3. Alcoholic fermentation, carried out by yeasts, produces carbon dioxide and ethyl alcohol; this process is used in the production of alcoholic spirits and breads. 4. Lactic acid fermentation, carried out by certain bacteria and fungi, produces lactic acid (lactate); this process is used commercially in the production of cheese, yogurt, and sauerkraut. 5. Other bacteria produce chemicals anaerobically, including isopropanol, butyric acid, proprionic acid, and acetic acid. Advantages and Disadvantages of Fermentation 1. Despite a low yield of two ATP molecules, fermentation provides a quick burst of ATP energy for muscular activity. 2. Lactate is toxic to cells. a. When blood cannot remove all lactate from muscles, lactate changes pH and causes muscles to fatigue. b. The individual is in oxygen debt because oxygen is needed to restore ATP levels and rid the body of lactate. c. Recovery occurs after lactate is sent to the liver where it is converted into pyruvate; some pyruvate is then respired or converted back into glucose. Efficiency of Fermentation 1. Two ATP produced per glucose molecule during fermentation is equivalent to 14.6 kcal. 7. E. 8.4 A. B. 49 2. 3. Complete glucose breakdown to CO2 and H2O during cellular respiration represents a potential yield of 686 kcal of energy. Efficiency of fermentation is 14.6/686 or about 2.1%, far less efficient than complete breakdown of glucose. 8.5 Metabolic Pool 1. Degradative reactions (catabolism) break down molecules; they tend to be exergonic. 2. Synthetic reactions (anabolism) build molecules; they tend to be endergonic. A. Catabolism 1. Just as glucose is broken down in cellular respiration, other molecules in the cell undergo catabolism. 2. Fat breaks down into glycerol and three fatty acids. a. Glycerol is converted to G3P, a metabolite in glycolysis. b. An 18-carbon fatty acid is converted to nine acetyl-CoA molecules that enter the citric acid cycle. c. Respiration of fat products can produce 108 kcal in ATP molecules; fats are an efficient form of stored energy. 3. Amino acids break down into carbon chains and amino groups. a. Hydrolysis of proteins results in amino acids. b. R-group size determines whether carbon chain is oxidized in glycolysis or the citric acid cycle. c. A carbon skeleton is produced in the liver by removal of the amino group, by the process of deamination. d. The amino group becomes ammonia (NH3), which enters the urea cycle and ultimately becomes part of excreted urea. e. The size of the R-group determines the number of carbons left after deamination. B. Anabolism 1. ATP produced during catabolism drives anabolism. 2. Substrates making up pathways can be used as starting materials for synthetic reactions. 3. The molecules used for biosynthesis constitute the cell’s metabolic pool. 4. Carbohydrates can result in fat synthesis: G3P converts to glycerol, acetyl groups join to form fatty acids. 5. Some metabolites can be converted to amino acids by transamination, the transfer of an amino acid group to an organic acid. 6. Plants synthesize all the amino acids they need; animals lack some enzymes needed to make some amino acids. 7. Humans synthesize 11 of 20 amino acids; the remaining 9 essential amino acids must be provided by the diet. Critical Thinking Question 1. Cyanide interrupts the cytochrome system of electron transport. Why is cyanide a universal poison effective in all organisms with mitochondria? Answer: The electron transport system is the producer of ATP from ADP in aerobic respiration. Stopping the electron transport system stops ATP production, which stops metabolic reactions, and this is essentially a universal system. Question 2. Breaking apart a molecule through combination with oxygen is burning. However, this is a “slow burn” inside of cells. Today, we hear of “spontaneous human combustion” where people allegedly burn up from runaway metabolism. Why is it impossible for rapid direct oxidation to originate in the cell environment? 50 Answer: Fire requires fuel, an abundant supply of gaseous oxygen, and a kindling temperature. The body does have fats, sugars, and other good fuel molecules. However, cells are 7090 percent water, oxygen is sparse and dissolved in this fluid, and the kindling temperature is far beyond the body temperatures that permit life or even boiling! In addition, the cellular respiration process described in this chapter shows how the breakdown of the glucose molecule is slowed by stages located in various parts of the cell and by metabolic pathways that take time, spreading out the “burn” so the released energy can be harvested for ATP rather than lost as heat. 51 CHAPTER 7.1 MITOSIS Chapter Outline 7.1 The Cell Cycle 1. The cell cycle is an orderly set of stages from the first division to the time the daughter cells divide. 2. When a cell is preparing for division, it grows larger, the number of organelles doubles, and the DNA replicates. A. Interphase 1. Most of a cell’s life is spent in interphase, in which the cell performs its usual functions. 2. Time spent in interphase varies by cell type: nerve and muscle cells do not complete the cell cycle and remain in the G0 stage while embryonic cells complete the cycle every few hours. 3. The G1 stage is just prior to DNA replication; a cell grows in size, organelles increase in number, and material accumulates for DNA synthesis. 4. The S stage is the DNA synthesis (replication) period; proteins associated with DNA are also synthesized; at the end of the S stage, each chromosome has two identical DNA double helix molecules, called sister chromatids. 5. The G2 stage occurs just prior to cell division; the cell synthesizes proteins needed for cell division, such as proteins in microtubules. 6. Interphase therefore consists of G1, S, and G2. B. M (Mitotic) Stage 1. M stage (M = mitosis) is the entire cell division stage, including both mitosis and cytokinesis. 2. Mitosis is nuclear division, cytokinesis is division of the cytoplasm. 3. When division of the cytoplasm is complete, two daughter cells are produced. C. Control of the Cell Cycle 1. The cell cycle is controlled by both internal and external signals. 2. A signal is a molecule that either stimulates or inhibits a metabolic event. 3. Growth factors are external signals received at the plasma membrane. 4. Cell Cycle Checkpoints a. There appear to be checkpoints where the cell cycle either stops or continues onward, depending on the internal signals it receives. D. Apoptosis 1. Apoptosis is programmed cell death and involves a sequence of cellular events. 2. Death by apoptosis prevents a tumor from developing. 7.2 Mitosis and Cytokinesis A. Eukaryotic Chromosomes 1. DNA in chromosomes of eukaryotic cells is associated with proteins; histone proteins organize chromosomes. 2. When a cell is not undergoing division, DNA in the nucleus is a tangled mass of threads called chromatin. 3. At cell division, chromatin becomes highly coiled and condensed and is now visible as individual chromosomes. 4. Each species has a characteristic number of chromosomes. a. The diploid (2n) number includes two sets of chromosomes of each type. 52 1) The diploid number is found in all the non-sex cells of an organism's body (with a few exceptions). 2) Examples include humans (46), crayfish (200), etc. b. The haploid (n) number contains one of each kind of chromosome. 1) In the life cycle of many animals, only sperm and egg cells have the haploid number. 2) Examples include humans (23), crayfish (100), etc. 5. Cell division in eukaryotes involves nuclear division and cytokinesis. a. Somatic cells undergo mitosis for development, growth, and repair. 1) This nuclear division leaves the chromosome number constant. 2) A 2n nucleus replicates and divides to provide daughter nuclei that are also 2n. b. A chromosome begins cell division with two sister chromatids. 1) Sister chromatids are two strands of genetically identical chromosomes. 2) At the beginning of cell division, they are attached at a centromere, a region of constriction on a chromosome. 9. B. Stages of Mitosis 1. The centrosome, the main microtubule organizing center of the cell, divides before mitosis begins. 2. Each centrosome contains a pair of barrel-shaped organelles called centrioles. 3. The mitotic spindle contains many fibers, each composed of a bundle of microtubules. 4. Mitosis is divided into five phases: prophase, prometaphase, metaphase, anaphase, and telophase. 5. Prophase a. Nuclear division is about to occur: chromatin condenses and chromosomes become visible. b. The nucleolus disappears and the nuclear envelope fragments. c. Duplicated chromosomes are composed of two sister chromatids held together by a centromere; chromosomes have no particular orientation in the cell at this time. 6. Prometaphase (Late Prophase) a. Specialized protein complexes (kinetochores) develop on each side of the centromere for future chromosome orientation. b. An important event during prometaphase is attachment of the chromosomes to the spindle and their movement as they align at the metaphase plate (equator) of the spindle. 7. Metaphase a. Chromosomes, attached to kinetochore fibers, are now aligned at the metaphase plate. 8. Anaphase a. The two sister chromatids of each duplicated chromosome separate at the centromere. Telophase a. Spindle disappears in this stage. b. The nuclear envelope reforms around the daughter chromosomes. c. The nucleolus reappears in each daughter nucleus. C. Cytokinesis in Animal and Plant Cells 53 1. Cytokinesis in Animal Cells A cleavage furrow indents the plasma membrane between the two daughter nuclei at a midpoint; this deepens to divide the cytoplasm during cell division. 2. D. 1. 2. 3. 4. Cytokinesis in Plant Cells a. The rigid cell wall that surrounds plant cells does not permit cytokinesis by furrowing. b. Vesicles fuse forming a cell plate; their membranes complete the plasma membranes of the daughter cells. Stem Cells Many mammalian organs contain stem cells (or adult stem cells), which retain the ability to divide. Red bone marrow stem cells repeatedly divide to produce the various types of blood cells. Therapeutic cloning to produce human tissues can begin with either adult stem cells or embryonic stem cells. Embryonic stem cells can be used for reproductive cloning, the production of a new individual. 7.3 The Cell Cycle and Cancer 1. Cancer is a cellular growth disorder that results from the mutation of genes that regulate the cell cycle; i.e., cancer results from the loss of control and a disruption of the cell cycle. 2. Carcinogenesis, the development of cancer is gradual—it may take decades before a cell has the characteristics of a cancer cell. Critical Thinking Question 1. Human red blood cells develop in the bone marrow from stem cells, and lose their nucleus before being released into the bloodstream. While this gives a cell that can be densely packed with hemoglobin molecules, what are the consequences as far as the longevity of the cell and its ability to replicate? Question 2. In some birds and true bugs, the number of chromosomes is hard to determine since chromosomes get smaller and smaller until they are too small to see. Yet, in animals it is rare to find chromosomes numbering over a hundred pairs. What is the probable reason for keeping chromosome numbers low? 54 CHAPTER 7.5 MEIOSIS AND SEXUAL REPRODUCTION Chapter Outline 7.4 Halving the Chromosome Number 1. Meiosis is nuclear division, reducing the chromosome number from the diploid (2n) to the haploid (n) number. 2. The haploid (n) number is half of the diploid number of chromosomes. 3. Sexual reproduction requires gamete (reproductive cell, often sperm and egg) formation and then fusion of gametes to form a zygote. 4. A zygote always has the full or diploid (2n) number of chromosomes. A. Homologous Pairs of Chromosomes 1. In diploid body cells, chromosomes occur as pairs. a. Each set of chromosomes is a homologous pair; each member is a homologous chromosome or homologue. b. Homologues look alike, have the same length and centromere position, and have a similar banding pattern when stained. c. A location on one homologue contains gene for the same trait that occurs at this locus on the other homologue, although the genes may code for different variations of that trait; alternate forms of a gene are called alleles. 2. Chromosomes duplicate immediately prior to nuclear division. a. Duplication produces two identical parts called sister chromatids; they are held together at the centromere. 3. One member of each homologous pair is inherited from the male parent, the other member from the female parent. 4. One member of each homologous pair will be placed in each sperm or egg. B. Overview of Meiosis 1. Meiosis involves two nuclear divisions and produces four haploid daughter cells. 2. Each daughter cell has half the number of chromosomes found in the diploid parent nucleus. 3. Meiosis I is the nuclear division at the first meiotic division. a. Prior to meiosis I, DNA replication occurs, each chromosome thus has two sister chromatids. b. During meiosis I, homologous chromosomes pair; this is called synapsis. c. During synapsis, the two sets of paired chromosomes lay alongside each other as a bivalent (sometimes called a tetrad). 4. In meiosis II, the centromeres divide and daughter chromosomes (derived as sister chromatids) separate. 7.5 Genetic Variation A. Genetic Recombination B. Independent assortment of homologous choromosomes 55 1. During independent assortment, the homologous chromosomes separate independently or in a random manner. 2. Independent assortment in a cell with only three pairs of chromosomes is 2 3 or eight combinations of maternal and paternal chromosomes. 3. In humans with 23 pairs of chromosomes, the combinations possible are 2 23 or 8,388,608, and this does not consider the variation from crossing-over. C. Fertilization 1. When gametes fuse at fertilization, chromosomes donated by parents combine. 2. The chromosomally different zygotes from same parents have (223)2 or 70,368,744,000,000 combinations possible without crossing-over. 3. If crossing-over occurs once, then (423)2 or 4,951,760,200,000,000,000,000,000,000 genetically different zygotes are possible for one couple. 7.6 The Phases of Meiosis A. Prophase I 1. Nuclear division is about to occur: nucleolus disappears; nuclear envelope fragments; centrosomes migrate away from each other; and spindle fibers assemble. 2. Homologous chromosomes undergo synapsis to form bivalents; crossing-over may occur at this time in which case sister chromatids are no longer identical. 3. Chromatin condenses and chromosomes become microscopically visible. B. Metaphase I C. Anaphase I D. Telophase I E. Meiosis II Much like mitosis 7.7 Meiosis Compared to Mitosis 1. 2. 3. Meiosis requires two nuclear divisions; mitosis requires only one nuclear division. Meiosis produces four daughter nuclei and four daughter cells; mitosis produces only two. The daughter cells produced by meiosis are haploid; the daughter cells produced by mitosis are diploid. 4. The daughter cells produced by meiosis are not genetically identical; the daughter cells produced by mitosis are genetically identical to each other and to the parental cell. Occurrence 1. In humans, meiosis occurs only in reproductive organs to produce gametes. 2. Mitosis occurs in all tissues for growth and repair. Critical Thinking Question 1. Meiosis, or duplication-division-division, is not the only way to reduce chromosome numbers by half. It is theoretically possible to simply divide the original diploid number of chromosomes to produce two haploid cells, and there is reportedly a primitive organism that does this. What would be a drawback? Question 2. Bees and ants have a haploid-diploid system for determining the sex of offspring. The queen can withhold sperm in her seminal receptacle and the unfertilized egg develops into a female. One species of ant has just two chromosomes in the diploid male and one in the haploid female. What effect would such a low chromosome number have on the standard “advantages” of sexual reproduction? 56 57 CHAPTER 8 MENDELIAN PATTERNS OF INHERITANCE 8.1 Gregor Mendel 1. Mendel was an Austrian monk. 2. Mendel formulated two fundamental laws of heredity in the early 1860s. 3. He arrived at a particulate theory of inheritance because it is based on the existence of minute particles—now called genes. 8.2 Mendel’s Law of Segregation 1. Mendel confirmed that his tall plants always had tall offspring, i.e., were true-breeding, before crossing two different strains that differed in only one trait—this is called a monohybrid cross. 2. A monohybrid cross is between two parent organisms true-breeding for two distinct forms of one trait. 3. Mendel tracked each trait through two generations. a. P generation is the parental generation in a breeding experiment. b. F1 generation is the first-generation offspring in a breeding experiment. c. F2 generation is the second-generation offspring in a breeding experiment. 4. He performed reciprocal crosses, i.e. pollen of tall plant to stigma of short plant and vice versa. 5. 6. 7. 8. His results were contrary to those predicted by a blending theory of inheritance. He found that the F1 plants resembled only one of the parents. Characteristics of other parent reappeared in about 1/4 of F 2 plants; 3/4 of offspring resembled the F1 plants. Mendel saw that these 3:1 results were possible if: a. F1 hybrids contained two factors for each trait, one being dominant and the other recessive; b. factors separated when gametes were formed; a gamete carried one copy of each factor; c. and random fusion of all possible gametes occurred upon fertilization. 9. Results of his experiments led Mendel to develop his first law of inheritance—the law of segregation: a. b. c. d. Each organism contains two factors for each trait. Factors segregate in the formation of gametes. Each gamete contains one factor for each trait. Fertilization gives each new individual two factors for each trait. A. As Viewed by Modern Genetics 1. Each trait in a pea plant is controlled by two alleles, alternate forms of a gene that occur at the same gene locus on homologous chromosomes. 2. A dominant allele masks or hides expression of a recessive allele; it is represented by an uppercase letter. 3. A recessive allele is an allele that exerts its effect only in the homozygous state; its expression is masked by a dominant allele; it is represented by a lowercase letter. 4. The gene locus is the specific location of alleles on homologous chromosomes. 5. 6. The process of meiosis explains Mendel’s law of segregation. In Mendel’s cross, the parents were true-breeding; each parent had two identical alleles for a 58 trait–they were homozygous, indicating they possess two identical alleles for a trait. 7. Homozygous dominant genotypes possess two dominant alleles for a trait. 8. Homozygous recessive genotypes possess two recessive alleles for a trait. 9. After cross-pollination, all individuals of the F1 generation had one of each type of allele. 10. Heterozygous genotypes possess one of each allele for a particular trait. 11. The allele not expressed in a heterozygote is a recessive allele. B. Genotype Versus Phenotype 1. Two organisms with different allele combinations can have the same outward appearance (e.g., TT and Tt pea plants are both tall; therefore, it is necessary to distinguish between alleles present and the appearance of the organism). 2. Genotype refers to the alleles an individual receives at fertilization (dominant, recessive). 3. Phenotype refers to the physical appearance of the individual (tall, short, etc.). C. One-trait Genetics Problems 1. First determine which characteristic is dominant; then code the alleles involved. 2. Determine the genotype and gametes for both parents; an individual has two alleles for each trait; each gamete has only one allele for each trait. 3. Each gamete is haploid; each has a 50% chance of receiving either allele. D. Laws of Probability 1. Probability is the likely outcome a given event will occur from random chance. a. For example, with every coin flip there is a 50% chance of heads and 50% chance of tails. b. Chance of inheriting one of either two alleles from a parent is also 50%. 2. The multiplicative law of probability states that the chance of two or more independent events occurring together is the product of the probability of the events occurring separately. a. The chance of inheriting a specific allele from one parent and a specific allele from another is ½ x ½ or 1/4. b. Possible combinations for the alleles Ee of heterozygous parents are the following: EE = ½ x ½ = 1/4 eE = ½ x ½ = 1/4 Ee = ½ x ½ = 1/4 ee = ½ x ½ = 1/4 3. The additive law of probability calculates the probability of an event that occurs in two or more independent ways; it is the sum of individual probabilities of each way an event can occur; in the above example where unattached earlobes are dominant (EE, Ee, and eE), the chance for unattached earlobes is 1/4 + 1/4 + 1/4 = 3/4. E. The Punnett Square 1. The Punnett square was introduced by R. C. Punnett (early 1900s) and provides a simple method to calculate the probable results of a genetic cross. 2. In a Punnett square, all possible types of sperm alleles are lined up vertically and all possible egg alleles are lined up horizontally; every possible combination is placed in squares. 3. The larger the sample size examined, the more likely the outcome will reflect predicted ratios; a large number of offspring must be counted to observe the expected results; only in that way can all possible genetic types of sperm fertilize all possible types of eggs. 4. Specific crosses in humans cannot be done in order to count many offspring; therefore in humans, the phenotypic ratio is used to estimate the probability of any child having a particular characteristic. 5. Punnett square uses laws of probability; it does not dictate what the next child will inherit. 6. “Chance has no memory”: if two heterozygous parents have a first child with attached earlobes (likely in 1/4th of children), a second child born still has 1/4 chance of having attached earlobes. F. One-Trait Testcross 1. To confirm that the F1 was heterozygous, Mendel crossed his F1 plants with homozygous recessive plants. 2. Results indicated the recessive factor was present in the F1 plants; they were thus 59 heterozygous. A monohybrid testcross is used between an individual with dominant phenotype and an individual with a recessive phenotype to see if the individual with dominant phenotype is homozygous or heterozygous. 8.3 Mendel’s Law of Independent Assortment 1. This two-trait (dihybrid) cross is between two parent organisms that are true-breeding for different forms of two traits; it produces offspring heterozygous for both traits. 2. Mendel observed that the F1 individuals were dominant in both traits. 3.. He further noted four phenotypes among F2 offspring; he deduced second law of heredity. 4. Mendel’s law of independent assortment states that members of one pair of factors assort independently of members of another pair, and that all combinations of factors occur in gametes. A. Two-trait Genetics Problems 1. Laws of probability indicate a 9:3:3:1 phenotypic ratio of F2 offspring resulting in the following: a. 9/16 of the offspring are dominant for both traits; b. 3/16 of the offspring are dominant for one trait and recessive for the other trait; c. 3/16 of the offspring are dominant and recessive opposite of the previous proportions; and d. 1/16 of the offspring are recessive for both traits. 2. The Punnett Square for two-trait crosses 3. a. A larger Punnett square is used to calculate probable results of this cross. b. A phenotypic ratio of 9:3:3:1 is expected when heterozygotes for two traits are crossed and simple dominance is present for both genes. c. Independent assortment during meiosis explains these results. B. Two-Trait Testcross 1. A two-trait testcross tests if individuals showing two dominant characteristics are homozygous for both or for one trait only, or heterozygous for both. 2. If an organism heterozygous for two traits is crossed with another recessive for both traits, the expected phenotypic ratio is 1:1:1:1. 3. In dihybrid genetics problems, the individual has four alleles, two for each trait. 8.4 Human Genetic Disorders A. Patterns of Inheritance 1. Genetic disorders are medical conditions caused by alleles inherited from parents. 2. An autosome is any chromosome other than a sex (X or Y) chromosome. 3. In a pedigree chart, males are designated by squares, females by circles; shaded circles and squares are affected individuals; line between square and circle represents a union; vertical line leads to offspring. 4. A carrier is a heterozygous individual with no apparent abnormality but able to pass on an allele for a recessively-inherited genetic disorder. 5. Autosomal dominant and autosomal recessive alleles have different patterns of inheritance. a. Characteristics of autosomal dominant disorders 1) Affected children usually have an affected parent. 2) Heterozygotes are affected.: two affected parents can produce unaffected child; two unaffected parents will not have affected children. b. Characteristics of autosomal recessive disorders 1) Most affected children have normal parents since heterozygotes have a normal phenotype. 2) Two affected parents always produce an affected child. 3) Close relatives who reproduce together are more likely to have affected children. 60 B. Autosomal Recessive Disorders 1. Tay-Sachs Disease a. Usually occurs among Jewish people in the U.S. of central and eastern European descent. b. Symptoms are not initially apparent; infant’s development begins to slow between four to eight months, neurological and psychomotor difficulties become apparent, child gradually becomes blind and helpless, develops seizures, eventually becomes paralyzed and dies by age of three or four. c. This results from lack of enzyme hexosaminidase A (Hex A) and the subsequent storage of its substrate, glycosphingolipid, in lysosomes. d. Primary sites of storage are cells of the brain; accounts for progressive deterioration. e. There is no treatment or cure. f. Prenatal diagnosis is possible by amniocentesis or chorionic villi sampling. g. The gene is located on chromosome 15. 2. Cystic Fibrosis a. This is the most common lethal genetic disease in Caucasians in the U.S. b. About 1 in 20 Caucasians is a carrier, and about 1 in 3,000 newborns has this disorder. c. An increased production of a viscous form of mucus in the lungs and pancreatic ducts is seen. 1) The resultant accumulation of mucus in the respiratory tract interferes with gas exchange. 2) Digestive enzymes must be mixed with food to supplant the pancreatic juices. d. New treatments have raised the average life expectancy to up to 35 years. e. Chloride ions (Cl–) fail to pass plasma membrane proteins. f. Since water normally follows Cl–, lack of water in the lungs causes thick mucus. g. The cause is a gene on chromosome 7; attempts to insert the gene into nasal epithelium has had little success. h. Genetic testing for adult carriers and fetuses is possible. 3. Phenylketonuria (PKU) a. PKU occurs once in every 5,000 births; it is the most common inherited disease of the nervous system. b. It is caused by a lack of an enzyme needed to metabolize amino acid phenylalanine; this results in accumulation of the amino acid in nerve cells of the brain and impairs nervous system development. c. PKU is caused by a gene on chromosome 12. d. Newborns are routinely tested in the hospital for high levels of phenylalanine in the blood. e. If an infant has PKU, the child is placed on a diet low in phenylalanine until the brain is fully developed, near age seven. 4. Sickle-Cell Disease a. This disease is the most common inherited disorder in blacks, affecting about 1 in 500 African Americans. 61 b. c. d. e. f. g. h. i. The gene is on chromosome 11. In affected individuals, the red blood cells are shaped like sickles—an abnormal hemoglobin molecule, Hbs, causes the defect. 1) Normal hemoglobin, HbA, differs from Hbs by one amino acid in the protein globin. The disease is an example of pleiotropy, describing a gene that affects more than one characteristic of an individual. Sickling of the red blood cells occurs when the oxygen content of the person’s blood is low, thereby slowing down blood flow and clogging small vessels. Signs and symptoms include anemia, weakness, fever, pain, rheumatism, low resistance to disease, kidney and heart failure. Treatment includes pain management, blood transfusions, and bone marrow transplants. The disease can be diagnosed prenatally. Individuals with the sickle cell trait (carriers), who normally do not have any sickle-shaped cells unless they experience dehydration or mild oxygen deprivation, are resistant to the disease malaria. C. Autosomal Dominant Disorders 1. Neurofibromatosis a. This is an autosomal dominant disorder that affects one in 3,500 newborns and is distributed equally around the world. b. Affected individuals have tan skin spots at birth, which develop into benign tumors. c. Neurofibromas are lumps under the skin comprised of fibrous coverings of nerves. d. In most cases, symptoms are mild and patients live a normal life; sometimes symptoms are severe: 1) skeletal deformities, including a large head; 2) eye and ear tumors that can lead to blindness and hearing loss; and 3) learning disabilities and hyperactivity. 4) Such variation is called variable expressivity. e. The gene that codes for neurofibromatosis was discovered in 1990 to be on chromosome 17. 1) The gene controls production of neurofibromin protein that normally blocks growth signals for cell division. 2) Many types of mutations result in this effect. 3) Some mutations are caused by a gene that moves from another location in the genome. 2. Huntington Disease a. This leads to progressive degeneration of brain cells, which in turn causes severe muscle spasm, personality disorders, and death in 10–15 years after onset. b. Most appear normal until they are of middle age and already have had children who might carry the gene; occasionally, first signs of the disease are seen in teenagers or even younger. 62 c. The gene for Huntington disease is located on chromosome 4. d. This gene contains many repeats of a base triplet that codes for glutamine in the huntingtin protein; normal persons have 10–15 glutamines; affected persons have 36 or more. e. A huntingtin protein with over 36 glutamines changes shape and forms large clumps inside neurons; it also attracts other proteins to clump with it. 3. Achondroplasia a. This disease is a common form of dwarfism, associated with a defect in the growth of long bones. b. Affected individuals have short arms and legs, a sway back, and a normal torso and head. c. About 1 in 25,000 people have the disease. d. Individuals with the disease are heterozygotes (Aa); the homozygous recessive (aa) condition yields normal-length limbs, while the homozygous dominant (AA) condition is lethal. 8.5 Beyond Mendelian Genetics A. Incomplete dominance: offspring show traits intermediate between two parental phenotypes. True-breeding red and white-flowered four-o’clocks produce pinkflowered offspring. B. Human Examples of Incomplete Dominance 1. Curly versus Straight Hair a. A curly-haired Caucasian and a straight-haired Caucasian will have wavyhaired offspring. b. Two wavy-haired parents will produce a 1:2:1 ratio of curly-wavy-straight hair children. 2. Sickle-cell disease, Tay Sachs disease, and cystic fibrosis are considered examples of incomplete dominance. C. Multiple Allelic Traits 1. This occurs when a gene has many allelic forms or alternative expressions. 2. ABO Blood Types a. The ABO system of human blood types is a multiple allele system. b. Two dominant alleles (IA and IB) code for presence of A and B glycoproteins on red blood cells. c. This also includes a recessive allele (iO) coding for no A or B glycoproteins on red blood cells. d. As a result, there are four possible phenotypes (blood types): A, B, AB, and O e. This is a case of codominance, where both alleles are fully expressed. 3. The Rh factor is inherited independently from the ABO system; the Rh+ allele is dominant. D. Polygenic Inheritance 1. Polygenic inheritance occurs when one trait is governed by two or more sets of alleles. 2. Dominant alleles have a quantitative effect on the phenotype: each adds to the effect. 3. The more genes involved, the more continuous is the variation in phenotypes, resulting in a bell-shaped curve. 4. Crosses of white and dark-red wheat seeds produce seeds with seven degrees 63 of intermediate colors due to genes at three separate loci. 5. Human Examples of Polygenic Inheritance a. A hybrid cross for skin color provides a range of intermediates. b. Parents with intermediate skin color can produce children with the full range of skin colors. c. Albinism, where one gene interferes with the expression of others, is an example of epistasis. E. Polygenic Disorders 1. This includes cleft lip, clubfoot, congenital dislocations of the hip, hypertension, diabetes, schizophrenia, allergies and cancers. 2. Behavioral traits including suicide, phobias, alcoholism, and homosexuality may be associated with particular genes but are not likely completely predetermined. 3. Environment and the Phenotype a. In water buttercups, the aquatic environment dramatically influences the structure of the plant. b. Temperature triggers a primrose to develop white flowers when grown above 32oC and red flowers when grown at 24oC. c. The coats of Siamese cats and Himalayan rabbits have darker tipped ears, nose, paws, etc. due to the enzyme encoded by an allele which is only active at the extremities at low temperatures. F. Environment and the Phenotype 1. Both genotype and the environment affect the phenotype. 2. Water and temperature can have profound influence on the phenotype. a. A flower might be one color at one temperature and another color at another temperature. b. The coat color of certain animals can change with temperature. Critical Thinking Question 1. Consider the proposal that handedness is inherited with right-handedness dominant (RR, Rr) and left handedness recessive (rr). Of all the possible combinations of parents and offspring by phenotype, what combination of parents and offspring would cast doubt on this simple hypothesis? Question 2. Corn has 10 pairs or 20 total chromosomes. If Mendel chose corn instead of pea plants to conduct his first experiments, what is the maximum number of traits he could have documented in breeding experiments in order to establish his law of segregation (each trait is determined by two factors) and independent assortment (the factors assort independent of other factors)? Question 3. Malaria has not been a serious health problem in the southern United States this century, but it remains a serious disease in Africa today. If we examined just the African American population descended from Africans brought to the United States from the malaria regions of Africa, how would the proportion of sickle-cell genes in these gene pools compare? What would occur to these gene frequencies over time if there was a resurgence of malaria in the United States? 64 CHAPTER 9 DNA: STRUCTURE AND FUNCTIONS Chapter Outline 8.1 The Genetic Material Early researchers knew that the genetic material must be: 1. able to store information used to control both the development and the metabolic activities of cells; 2. stable so it can be replicated accurately during cell division and be transmitted for generations; and, 3. able to undergo mutations providing the genetic variability required for evolution. Previous Knowledge About DNA 1. Understanding the chemistry of DNA was essential to the discovery that DNA is genetic material. 2. Friedrich Miescher (1869) removed nuclei from pus cells and isolated DNA “nuclein”; it was rich in phosphorus and lacked sulfur. 3. Subsequent analysis of nuclein found that it contained an acidic substance: named it nucleic acid. 4. Two types of nucleic acids were soon discovered: DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). 5. In the early twentieth century, it was shown that nucleic acids contain four types of nucleotides. a. DNA was composed of repeating units, each of which always had just one of each of four different nucleotides (a nitrogenous base, a phosphate, and a pentose). b. In this model, DNA could not vary between species and therefore could not be the genetic material; therefore some other protein component was expected to be the genetic material. A. Transformation of Bacteria 1. Bacteriologist Frederick Griffith (1931) experimented with Streptococcus pneumoniae (a pneumococcus that causes pneumonia in mammals). 2. Mice were injected with two strains of pneumococcus: an encapsulated (S) strain and a nonencapsulated (R) strain. a. The S strain is virulent (the mice died); it has a mucous capsule and forms “shiny” colonies. b. The R strain is not virulent (the mice lived); it has no capsule and forms “dull” colonies. 3. In an effort to determine if the capsule alone was responsible for the virulence of the S strain, he injected mice with heat-killed S strain bacteria; the mice lived. 4. Finally, he injected mice with a mixture of heat-killed S strain and live R strain bacteria. a. The mice died; living S strain pneumococcus were recovered from their bodies. b. Griffith concluded that some substance necessary for synthesis of the capsule--and therefore for virulence--must pass from dead S strain bacteria to living R strain bacteria so the R strain were transformed. c. This change in phenotype of the R strain must be due to a change in the bacterial genotype, suggesting that the transforming substance passed from S strain to R strain. B. DNA: The Transforming Substance 1. Oswald Avery et al. (1944) reported that the transforming substance was DNA. 2. Purified DNA is capable of bringing about the transformation. Evidence: a. DNA from S strain pneumococcus causes R strain bacteria to be transformed. b. Enzymes that degrade proteins cannot prevent transformation, nor can enzymes that digest RNA. c. Digestion of the transforming substance with enzyme that digests DNA prevents 65 transformation. The molecular weight of the transforming substance is great enough for some genetic variability. 3. Avery’s experimental results demonstrated DNA is genetic material and DNA controls biosynthetic properties of a cell. C. Reproduction of Viruses 1. Bacteriophages are viruses that infect bacteria; they consist of a protein coat surrounding a nucleic acid. 2. Bacteriophage T2 is a virus that infects the Escherichia coli (E. coli), a species of bacteria that normally lives within the human gut. 3. Alfred Hershey and Martha Chase (1952) studied bacteriophage T2. a. The purpose of their experiments was to see which of the bacteriophage components— the protein coat or the DNA—entered bacterial cells and directed reproduction of the virus. b. They labeled the protein coat with 35S and the DNA with 32P. c. Viral coats were removed from the bacterial cells and separated by centrifugation. d. Results: radioactive 32P alone is taken up by bacterial host and incorporated in virus reproduction. e. This result reinforced the notion that DNA (and not the protein) is the genetic material. d. 8.2 The Structure of DNA A. Nucleotide Data 1. Erwin Chargaff (1940s) analyzed the base content of DNA. 2. It was known DNA contained four different nucleotides: a. two with purine bases, adenine (A) and guanine (G); a purine is a type of nitrogencontaining base having a double-ring structure. b. two with pyrimidine bases, thymine (T) and cytosine (C); a pyrimidine is a type of nitrogen-containing base having a single-ring structure. 3. Results: DNA does have the variability necessary for the genetic material, and, 4. For a species, DNA has the constancy required of genetic material. 5. This constancy is given in Chargaff’s rules: a. The amount of A, T, G, and C in DNA varies from species to species. b. In each species, the amount of A = T and the amount of G = C (A +G = T +C). 6. The tetranucleotide hypothesis (proposing DNA was repeating units of one of four bases) was disproved: each species has its own constant base composition. B. Variation in Base Sequence 1. The variability is staggering; a human chromosome contains about 140 million base pairs. 2. Since any of the four possible nucleotides can be present at each nucleotide position, the total 6 number of possible nucleotide sequences is 4140 x 10 = 4140,000,000. C. Diffraction Data 1. Rosalind Franklin produced X-ray diffraction photographs. 2. Franklin’s work provided evidence that DNA had the following features: a. DNA is a helix. b. Some portion of the helix is repeated. D. The Watson and Crick Model 1. American James Watson joined with Francis H. C. Crick in England to work on the structure of DNA. 2. Watson and Crick received the Nobel Prize in 1962 for their model of DNA. 3. Using information generated by Chargaff and Franklin, Watson and Crick constructed a model of DNA as a double helix with sugar-phosphate groups on the outside, and paired bases on the inside. 4. Their model was consistent with both Chargaff’s rules and Franklin’s X-ray diffraction studies. 5. Complementary base pairing is the paired relationship between purines and pyrimidines in DNA: A is hydrogen-bonded to T and G is hydrogen-bonded to C. 8.3 Replication of DNA: This process consists of: 66 1. Unwinding: old strands of the parent DNA molecule are unwound as weak hydrogen bonds between the paired bases are “unzipped” and broken by the enzyme helicase. 2. Complementary base pairing: free nucleotides present in the nucleus bind with complementary bases on unzipped portions of the two strands of DNA; this process is catalyzed by DNA polymerase. 3. Joining: complementary nucleotides bond to each other to form new strands; each daughter DNA molecule contains an old strand and a new strand; this process is also catalyzed by DNA polymerase. 4. DNA replication must occur before a cell can divide; in cancer, drugs with molecules similar to the four nucleotides are used to stop replication. A. Replication is Semiconservative 1. DNA replication is semiconservative: each daughter double helix has one parental strand and one new strand. 2. Matthew Meselson and Franklin Stahl (1958) confirmed the process of DNA replication. a. They grew bacteria in a medium with heavy nitrogen (15N), then switched to light nitrogen (14N). b. The density of DNA following replication is intermediate as measured by centrifugation of molecules. c. After one division, only “hybrid” DNA molecules were in the cells. d. After two divisions, half the DNA molecules were “light” and half were “hybrid.” 3. These were exactly the results to be expected if DNA replication is semiconservative. B. Eukaryotic Replication 1. Replication in eukaryotes starts at many points of origin and spreads with many replication bubbles—places where the DNA strands are separating and replication is occurring. 2. Replication forks are the V-shape ends of the replication bubbles; the sites of DNA replication. 3. Eukaryotes replicate their DNA at a slower rate – 500 to 5,000 base pairs per minute. 4. Eukaryotes take hours to complete DNA replication. C. Replication Errors 1. A genetic mutation is a permanent change in the sequence of bases. 2. Base changes during replication are one way mutations occur. 3. A mismatched nucleotide may occur once per 100,000 base pairs, causing a pause in replication. 4. Proofreading is the removal of a mismatched nucleotide; DNA repair enzymes perform this proofreading function and reduce the error rate to one per billion base pairs. 5. Incorrect base pairs that survive the proofreading process contribute to gene mutations. Critical Thinking Question 1. In what human cells would you find the highest concentration of DNA compared with other cell components? Question 2. As quartz crystals “grow,” new silicon dioxide molecules are aligned alongside the previous surfaces. Likewise, as aluminum silicates and other components of clay are dissolved and precipitate out, they too align with previous molecules, even perpetuating cracks and faults. Technically, these are self-replicating molecular assemblages and yet the quartz or clay has not risen to become “life.” What feature(s) of DNA makes it different from quartz and clay? 67 CHAPTER 10 GENE EXPRESSION AND CONTROL The history of the research recognizing and elaborating gene action is discussed. The processes of transcription and translation are described in detail, and are accompanied with detailed graphics. The chemical nature of the RNAs and their roles in gene expression are outlined. Chapter Outline 10.1 The Function of Genes A. Genes Specify Enzymes 1. George Beadle and Edward Tatum (1940) X-rayed spores of the red bread mold, Neurospora crassa. 2. They observed that some resulting cultures lacked a particular enzyme for growth on minimal medium. 3. They found that a single gene was mutated, which resulted in the lack of a single enzyme. 4. They proposed the one gene–one enzyme hypothesis: one gene specifies the synthesis of one enzyme. B. Genes Specify a Polypeptide 1. Linus Pauling and Harvey Itano (1949) compared hemoglobin in red blood cells of persons with sickle-cell disease and normal individuals. 2. They discovered that the chemical properties of a protein chain of sickle-cell hemoglobin differed from that of normal hemoglobin. 3. Vernon Ingram subsequently showed that the biochemical difference in the protein chain of sickle-cell hemoglobin is the substitution of a nonpolar amino acid valine for the negatively charged amino acid glutamate. 4. Pauling and Itano formulated the one gene–one polypeptide hypothesis: each gene specifies one polypeptide of a protein, a molecule that may contain one or more different polypeptides. C. From DNA to RNA to Protein 1. A gene is a sequence of DNA nucleotide bases that codes for a sequence of nucleotides in an RNA molecule. 2. DNA is restricted to nucleus; protein synthesis occurs at ribosomes in the cytoplasm. 3. Ribonucleic acid (RNA) is found in both regions of the cell. D. Types of RNA 1. Like DNA, RNA is a polymer of nucleotides. 2. Unlike DNA, RNA is single-stranded, contains the sugar ribose, and the base uracil instead of thymine (in addition to cytosine, guanine, and adenine). 3. There are three major classes of RNA. a. Messenger RNA (mRNA) takes a message from DNA in the nucleus to ribosomes in the cytoplasm. b. Ribosomal RNA (rRNA) and proteins make up ribosomes where proteins are synthesized. c. Transfer RNA (tRNA) transfers a particular amino acid to a ribosome. E. Gene Expression 1. DNA undergoes transcription to mRNA, which is translated to a protein. 2. DNA is a template for RNA formation during transcription. 3. Transcription is the first step in gene expression; it is the process whereby a DNA strand serves as a template for the formation of mRNA. 4. During translation, an mRNA transcript directs the sequence of amino acids in a polypeptide. 10.2 The Genetic Code 1. The central dogma of molecular biology states that the sequence of nucleotides in DNA specifies the order of amino acids in a polypeptide. 68 2. 3. 4. The genetic code is a triplet code, comprised of three-base code words (e.g., AUG). A codon consists of 3 nucleotide bases of DNA. Four nucleotides based on 3-unit codons allows up to 64 different amino acids to the specified. A. Finding the Genetic Code 1. Marshall Nirenberg and J. Heinrich Matthei (1961) found that an enzyme that could be used to construct synthetic RNA in a cell-free system; they showed the codon UUU coded for phenylalanine. 2. By translating just three nucleotides at a time, they assigned an amino acid to each of the RNA codons, and discovered important properties of the genetic code. 3. The code is degenerate: there are 64 triplets to code for 20 naturally occurring amino acids; this protects against potentially harmful mutations. 4. The genetic code is unambiguous; each triplet codon specifies one and only one amino acid. 5. The code has start and stop signals: there are one start codon and three stop codons. B. The Code Is Universal 1. The few exceptions to universality of the genetic code suggests the code dates back to the very first organisms and that all organisms are related. 2. Once the code was established, changes would be disruptive. 10.3 First Step: Transcription A. Messenger RNA is Formed 1. A segment of the DNA helix unwinds and unzips. 2. Transcription begins when RNA polymerase attaches to a promoter on DNA. A promoter is a region of DNA which defines the start of the gene, the direction of transcription, and the strand to be transcribed. 3. As RNA polymerase (an enzyme that speeds formation of RNA from a DNA template) moves along the template strand of the DNA, complementary RNA nucleotides are paired with DNA nucleotides of the coding strand. The strand of DNA not being transcribed is called the noncoding strand. 4. RNA polymerase adds nucleotides to the 3'-end of the polymer under construction. Thus, RNA synthesis is in the 5’-to-3’ direction. 5. The RNA/DNA association is not as stable as the DNA double helix; therefore, only the newest portion of the RNA molecule associated with RNA polymerase is bound to DNA; the rest dangles off to the side. 6. Elongation of mRNA continues until RNA polymerase comes to a stop sequence. 7. The stop sequence causes RNA polymerase to stop transcribing DNA and to release the mRNA transcript. 8. Many RNA polymerase molecules work to produce mRNA from the same DNA region at the same time. 9. Cells produce thousands of copies of the same mRNA molecule and many copies of the same protein in a shorter period of time than if a single copy of RNA were used to direct protein synthesis. B. RNA Molecules Are Processed 1. Newly formed primary mRNA transcript is processed before leaving the nucleus. 2. Primary mRNA transcript is the immediate product of transcription; it contains exons and introns. 3. The ends of the mRNA molecule are altered: a cap is put on the 5' end and a poly-A tail is put on the 3' end. a. The “cap” is a modified guanine (G) where a ribosome attaches to begin translation. b. The “poly-A tail” consists of a 150–200 adenine (A) nucleotide chain that facilitates transport of mRNA out of the nucleus and inhibits enzymatic degradation of mRNA. 4. Portions of the primary mRNA transcript, called introns, are removed. a. An exon is a portion of the DNA code in the primary mRNA transcript eventually expressed in the final polypeptide product. b. An intron is a non-coding segment of DNA removed by spliceosomes before the mRNA leaves the nucleus. 5. Ribozymes are RNAs with an enzymatic function restricted to removing introns from 69 themselves. a. RNA could have served as both genetic material and as the first enzymes in early life forms. 6. Spliceosomes are complexes that contains several kinds of ribonucleoproteins. a. Spliceosomes cut the primary mRNA transcript and then rejoin adjacent exons. C. Function of Introns 1. In humans, introns comprise 95% of the average protein-coding gene. 2. Possibly introns divide a gene into regions that can be joined in different combinations for different products. 3. Introns may function to determine which genes are to be expressed and how they should be spliced. 10.4 Second Step: Translation 1. Translation takes place in the cytoplasm of eukaryotic cells. 2. Translation is the second step by which gene expression leads to protein synthesis. 3. One language (nucleic acids) is translated into another language (protein). A. The Role of Transfer RNA 1. transfer RNA (tRNA) molecules transfer amino acids to the ribosomes. 2. The tRNA is a single-stranded ribonucleic acid that doubles back on itself to create regions where complementary bases are hydrogen-bonded to one another. 3. The amino acid binds to the 3’ end; the opposite end of the molecule contains an anticodon that binds to the mRNA codon in a complementary fashion. 4. There is at least one tRNA molecule for each of the 20 amino acids found in proteins. 5. There are fewer tRNAs than codons because some tRNAs pair with more than one codon; if an anticodon contains a U in the third position, it will pair with either an A or G–this is called the wobble hypothesis. 6. The tRNA synthetases are amino acid-activating enzymes that recognize which amino acid should join which tRNA molecule, and covalently joins them. This requires ATP. 7. An amino acid–tRNA complex forms, which then travels to a ribosome to “transfer” its amino acid during protein synthesis. B. The Role of Ribosomal RNA 1. Ribosomal RNA (rRNA) is produced from a DNA template in the nucleolus of the nucleus. 2. The rRNA is packaged with a variety of proteins into ribosomal subunits, one larger than the other. 3. Subunits move separately through nuclear envelope pores into the cytoplasm where they combine when translation begins. 4. Ribosomes can float free in cytosol or attach to endoplasmic reticulum. 5. Prokaryotic cells contain about 10,000 ribosomes; eukaryotic cells contain many times more. 6. Ribosomes have a binding site for mRNA and binding sites for two tRNA molecules. 7. They facilitate complementary base pairing between tRNA anticodons and mRNA codons; rRNA acts as an enzyme (ribozyme) that joins amino acids together by means of a peptide bond. 8. A ribosome moves down the mRNA molecule, new tRNAs arrive, the amino acids join, and a polypeptide forms. 9. Translation terminates once the polypeptide is formed; the ribosome then dissociates into its two subunits. 10. Polyribosomes are clusters of several ribosomes synthesizing the same protein. 11. To get from a polypeptide to a function protein requires correct bending and twisting; chaperone molecules assure that the final protein develops the correct shape. 12. Some proteins contain more than one polypeptide; they must be joined to achieve the final three-dimensional shape. C. Translation Requires Three Steps 1. During translation, mRNA codons base-pair with tRNA anticodons carrying specific amino acids. 2. Codon order determines the order of tRNA molecules and the sequence of amino acids in polypeptides. 3. Protein synthesis involves initiation, elongation, and termination. 70 4. 5. Enzymes are required for all three steps; energy (ATP) is needed for the first two steps. Chain Initiation a. A small ribosomal subunit attaches to mRNA in the vicinity of the start codon (AUG). b. First or initiator tRNA pairs with this codon; then the large ribosomal subunit joins to the small subunit. c. Each ribosome contains three binding sites: the P (for peptide) site, the A (for amino acid) site, and the E (for exit) site. d. The initiator tRNA binds to the P site although it carries one amino acid, methionine. e. The A site is for the next tRNA carrying the next amino acid. f. The E site is to discharge tRNAs from the ribosome. g. Initiation factor proteins are required to bring together the necessary translation components: the small ribosomal subunit, mRNA, initiator tRNA, and the large ribosomal subunit. 6. Chain Elongation a. The tRNA with attached polypeptide is at the P site; a tRNA-amino acid complex arrives at the A site. b. Proteins called elongation factors facilitate complementary base pairing between the tRNA anticodon and the mRNA codon. c. The polypeptide is transferred and attached by a peptide bond to the newly arrived amino acid in the A site. d. This reaction is catalyzed by a ribozyme, which is part of the larger subunit. e. The tRNA molecule in the P site is now empty. f. Translocation occurs with mRNA, along with peptide-bearing tRNA, moving to the P site and the spent tRNA moves from the P site to the E site and exits the ribosome. g. As the ribosome moves forward three nucleotides, there is a new codon now located at the empty A site. h. The complete cycle is rapidly repeated, about 15 times per second in Escherichia coli. 7. Chain Termination a. Termination of polypeptide synthesis occurs at a stop codon that does not code for amino acid. b. The polypeptide is enzymatically cleaved from the last tRNA by a release factor. c. The tRNA and polypeptide leave the ribosome, which dissociates into its two subunits. 8. Definition of a Gene and a Genetic Mutation a. Originally a gene was defined as a locus on the chromosome. b. The one gene-one polypeptide concept connected inborn errors of metabolism with a sequence of DNA bases. c. A gene could also be defined as a sequence of DNA bases coding for a single polypeptide or a single RNA. d. These concepts can allow us to define a mutation as a permanent change in the sequence of DNA bases. e. Current definitions: a protein-coding gene is one that is transcribed into mRNA, while a noncoding gene is one that is transcribed into any other type of RNA. D. Protein Synthesis and the Eukaryotic Cell 1. The first few amino acids of a polypeptide act as a signal peptide that indicates where the polypeptide belongs in the cell or if it is to be secreted by the cell. 2. After the polypeptide enters the lumen of the ER, it is folded and further processed by addition of sugars, phosphates, or lipids. 3. Transport vesicles carry the proteins between organelles and to the plasma membrane. 71 Critical Thinking Question 1. How can a cell produce the largest amount of coded protein product in the shortest time? Question 2. eukaryotes? If bacteria do not have introns and we have many, what is the likely status of simpler Question 3. Why would evolution not be able to select for “twins” for codons (instead of “triplets”); that is, only two base pairs would code for an amino acid? Question 4. The final product of gene activity is a protein. But cells are made of many other molecules also, including the phospholipid bilayer membrane, carbohydrate-derived structures such as cellulose, etc. Also the recent elaboration of the human genome has revealed far fewer genes than the diversity of proteins in the human body cells. How are these a result of gene activity? CHAPTER 17 AND 20 ANIMAL ORGANIZATION AND HOMEOSTASIS Chapter Outline 17-20.1 Types of Tissues A tissue is composed of specialized cells of the same type that perform a common function in the body. A. Four Major Types of Tissue 1. Epithelial tissue covers body surfaces and lines body cavities. 2. Connective tissue binds and supports body parts. 3. Muscular tissue causes body parts to move. 4. Nervous tissue responds to stimuli and transmits impulses. B. Epithelial Tissues 1. Epithelial tissue forms a continuous layer over the body surfaces including inner cavities. 2. Epithelial tissue cells are packed tightly C. Connective Tissue 1. Connective tissue binds structures together, provides support and protection, fills spaces, stores fat, and forms blood cells. 2. Connective tissue provides source cells for muscle and skeletal cells in animals that regenerate parts. 3. Connective tissue cells are separated widely by a matrix, a noncellular material between cells. tendons ligaments Adipose Tissue Cartilage and bone Fluid Connective Tissue: Blood D. Muscular Tissue 1 Skeletal muscle attaches by tendons to the bones of the skeleton. a. Skeletal muscle moves body parts, is under voluntary control, and contracts faster than other types. b. Skeletal muscle fibers are long, cylindrical, multinucleate cells arising from the fusion of 72 several cells. Skeletal fibers are striated due to the light and dark bands of overlapping actin and myosin filaments. Smooth (visceral) muscle is not striated. a. Spindle-shaped fibers form layers with the thick middle portion of one fiber opposite the thin ends of adjacent fibers. b. The nuclei form an irregular pattern in the tissue. c. Smooth muscle is not under voluntary control; it is therefore involuntary. d. Smooth muscle is found in the walls of viscera (e.g., intestine, stomach, etc.) and blood vessels. e. Smooth muscles drive the intestinal contractions and blood vessel constrictions. Cardiac muscle is found only in the heart wall and powers the heartbeat that pumps blood. a. Cardiac muscle combines the features of both smooth and skeletal muscle. b. Unlike skeletal muscles with many nuclei, cardiac muscles have one centrally placed nucleus. c. Although it appears to be one mass of muscle fibers, the cardiac muscle fibers are individual cells. d. Cardiac muscle cells are bound end-to-end at intercalated disks where the folded membranes between two fibers contain desmosomes and gap junctions e. Impulses move from cell to cell so the heartbeat is coordinated. c. 2. 3. E. Nervous Tissue: Nervous tissue contains neurons in the brain, spinal cord, and nerves. 73 17-20.2 Organs and Organ Systems Organs are combinations of two or more different tissues performing common functions. 1. An organ system contains many different organs that cooperate to carry out a process (e.g., digestion). 2. The integumentary system is composed of the skin and accessory organs (i.e., nails, hair, glands, and sensory receptors). A. Skin as an Organ 1. Human skin protects the underlying tissues from trauma, desiccation, radiation damage, and microbial invasion. 2. The skin produces a precursor molecule that is converted to vitamin D after exposure to UV light. 3. The skin also helps regulate body temperature. 4. Laden with sensory receptors, the skin collects information about the external environment. B. Regions of Skin 1. The skin has both an outer epidermal layer (epidermis) and a deeper layer (dermis); a subcutaneous layer (hypodermis) is found between the skin and underlying structures. 2. The epidermis is the outer, thinner layer of skin. a. Melanocytes located in the basal layer produce a melanin pigment that absorbs UV light, protecting deeper cells from radiation damage; certain cells in the epidermis convert a steroid related to cholesterol into vitamin D, a chemical required for proper bone growth. b. Skin Cancer 1) Too much ultraviolet radiation is dangerous and can lead to skin cancer. 2) Excessive exposure to UV radiation can convert cells in the basal layer of the epidermis into cancer cells (basal cell carcinoma); melanoma is skin cancer derived from melanocytes. 3. The dermis is fibrous connective tissue that forms a thicker and deeper layer of skin. a. The dermis contains both elastic fibers and collagen fibers; these run parallel with the skin surface. b. The dermis contains blood vessels that can constrict and dilate. c. Many small sensory receptors are present in the dermis. 1) There are separate receptors for pressure, touch, temperature, and pain. 4. The subcutaneous layer is not technically a part of the skin; it is composed of loose connnective tissue and adipose cells, which store fat. a. This is composed of loose connective tissue, including adipose tissue. b. Adipose tissue helps insulate the body by minimizing both heat gain and heat loss. This layer of adipose gives a rounded appearance to the body. C. Accessory Organs of the Skin 1. Nails grow from special epidermal cells at the base of the nail in a region called the nail root. 2. A hair follicle contains a nonliving hair shaft and the living hair root that produced it. a. The hair shaft is formed of dead, keratinized epidermal cells that protect the surface of the skin. b. The arrector pili muscle is a smooth muscle attached to the hair follicle; contracting it causes the hair to erect. c. Follicles have oil (sebaceous) glands producing sebum, an oil secreted to lubricate both the hair and the skin. 3. The sweat (sudoriferous) glands are coiled tubules present in most of the regions of skin that secrete a fluid (sweat) onto the surface of the skin. 17-20.3 Homeostasis The cells of the body live in an internal environment, tissue fluid that bathes the cells of an animal’s body. 1. This concept was first proposed by Claude Bernard, a famous French physiologist in 1859. 2. The internal environment (e.g., composition and temperature) must stay within normal range. 3. This relative internal stability allows animals to tolerate considerable external variation. 4. The American physiologist Walter Cannon first used the term “homeostasis.” 74 Homeostasis is the maintenance of internal conditions in a cell or organism by means of self-regulating mechanisms that curtail fluctuations above and below a normal range. 1. The organ systems of the human body contribute to homeostasis. a. The respiratory system adds oxygen and removes carbon dioxide; the amounts are altered to meet needs. b. The liver removes and stores glucose as glycogen and then replaces the blood glucose levels when they lower. c. The hormone insulin is secreted by the pancreas to regulate glucose levels. d. The kidneys are under hormonal control to excrete wastes and salts and to maintain blood pH. 2. Although homeostasis is controlled by hormones, it is ultimately controlled by the nervous system. 3. The brain contains centers that regulate temperature and blood pressure. 4. Regulation requires a receptor that detects unacceptable levels and signals a regulator center that can direct an adaptive response; once normalcy is obtained, the receptor is no longer stimulated. A. Negative Feedback 1. A negative feedback mechanism involves a response in which a variable is kept close to a particular set point. a. The process involves a sensor and a control center. b. The sensor detects a change in the internal environment. c. The control center brings about an effect to bring conditions back to normal. d. Example: the pancreas detects that the blood glucose level is too high; it secretes insulin which causes cells to take up glucose; blood glucose level returns to normal. e. A home heating system is a mechanical example of a negative feedback mechanism. f. Human Example: Regulation of Body Temperature 1) The sensor and control center are located in the hypothalamus. 2) When body temperature is above normal, the control center directs blood vessels in the skin to dilate—heat is lost to the environment. 3) When body temperature is below normal, the the control center directs blood vessels in the skin to constrict—heat is conserved in the body. B. Positive Feedback 1. A positive feedback mechanism involves output that intensifies and increases the input, thereby increasing the process; an ever-greater change in the same direction occurs. 2. Once childbirth begins, each event amplifies; the process continues until birth occurs. 3. Positive feedback mechanisms can be harmful, e.g., when a fever causes metobolic changes that push the fever even higher. Critical Thinking Question 1. Why would more skin pigment be selected in tropical regions and less skin pigment be preponderant in polar regions? Question 2. How do the feedback mechanisms of labor and childbirth differ from those of maintaining a constant body temperature? 75 CHAPTER 22.1 - 22.5 CIRCULATION AND CARDIOVASCULAR SYSTEMS 22.1 Transport in Invertebrates 1. 2. 3. Unicellular protozoa exchange directly with the environment across the plasma membrane. Some multicellular animals lack an internal transport system. The larger invertebrates usually have circulatory systems—either an open system or a closed system. A. Invertebrates Without a Circulatory System 1. Sea anemones and planaria are organisms with a saclike body plan that makes a circulatory system unnecessary. 2. Sea anemone cells are part of an external layer or gastrovascular cavity and diffusion supplies all the nutrients. 3. Planaria have a trilobed gastrovascular cavity and a small, flat body where nutrients diffuse from cell to cell. 4. Pseudocoelomates, such as nematodes, use the coelomic fluid of the body cavity to transport fluids. 5. Echinoderms rely on movement of coelomic fluid as a circulatory system. B. Invertebrates with an Open or a Closed System Circulatory System 1. In a circulatory system, a pumping heart moves one of two types of circulatory fluids. a. Blood is a circulatory fluid and is always contained within blood vessels b. Hemolymph is a circulatory fluid which flows into the hemocoel of certain arthropods and molluscs; it is a mixture of blood and interstitial fluid. 2. Certain arthropods and molluscs have an open circulatory system. a. Hemolymph is pumped by the heart into the body cavity or saclike sinuses. b. Hemolymph bathes the internal organs and then drains back to the heart. c. In grasshoppers, a dorsal heart pumps hemolymph into an aorta, which empties into the hemocoel. d. Hemolymph is colorless (it lacks hemoglobin or other respiratory pigments); a system of tracheae provides oxygen. 3. Some invertebrates, including earthworms and cephalopods, have a closed circulatory system in which blood never leaves the heart or vessels. a. Valves prevent any backward flow of the blood as it moves through vessels. b. Earthworms have five pairs of anterior lateral vessels that pump blood to every segment. c. Blood moves in capillaries where an exchange with tissue fluid takes place before returning in veins. d. Earthworms have a red respiratory pigment hemoglobin dissolved in the blood, not inside blood cells. e. With no special cavity for gas exchange, the gas must diffuse across a moist body wall. 22.2 Transport in Vertebrates 1. 2. Vertebrates have a closed circulatory system called a cardiovascular system. The muscular heart keeps blood circulating through the blood vessels. 76 a. The atria are the chambers of the heart that receive blood. b. The ventricles pump blood into arteries. 3. There are three kinds of blood vessels: arteries carry the blood away from the heart, capillaries are where the exchange with tissue fluid takes place, and veins return the blood to the heart. a. Arteries 1) have thick walls and are resilient. 2) expand to accommodate sudden increase in blood volume that results after heart contraction. 3) divide into small arterioles. b. Arteriole constriction and dilation is regulated by the nervous system to affect blood pressure. c. Capillaries are microscopic blood vessels with a wall formed of one layer of simple squamous cells. 1) Capillary beds are so prevalent that, in humans, all cells are within 60–80 µm of a capillary. 2) Only 5% of the capillaries are open at one time; after an animal has eaten, the capillary beds of the digestive system open. 3) Capillaries are so narrow that red blood cells must pass through them in single file. 4) Gas, nutrient, and waste exchange occurs across the thin capillary walls. d. The venules are vessels that take blood from capillaries and join to form a vein. e. Veins transport blood toward the heart. 1) The walls of a veins are much thinner than those of arteries; there is a lower blood pressure in veins than in arteries. 2) One-way valves open in the direction of the heart, then close to prevent backflow. A. Comparison of Circulatory Pathways 1. In vertebrates, there are three different types of circulatory pathways. 2. Fishes have a one-circuit (single-loop) circulatory pathway. a. The heart has a single atrium and a single ventricle and pumps the blood under pressure to the gills. b. Blood is oxygenated in the gills. c. After passing through gills, blood is returned to the dorsal aorta, which distributes the blood throughout the body. 3. Other vertebrates have a two-circuit (double-loop) circulatory pathway to breathe air on land. a. The systemic circuit transports the blood to tissues. b. The pulmonary circuit pumps the blood to lungs. 4. In amphibians and most reptiles, the heart has two atria and a single ventricle. 5. In most reptiles, and in all birds and mammals, the heart is divided into left and right halves. a. With two atria and two ventricles, the oxygenated blood is always separate from the deoxygenated blood. b. The right ventricle pumps blood to the lungs; the left ventricle pumps blood to the rest of the body. c. This arrangement provides adequate blood pressure for both the pulmonary and the systemic circulations. 22.3 Transport in Humans A. The Human Heart 1. The pumping of the heart keeps the blood moving in arteries. 2. Skeletal muscle contraction is responsible for the blood movement in veins. 3. The heart is a cone-shaped, muscular organ about the size of a fist. 4. It is located between the lungs directly behind the sternum and is tilted so that the apex is oriented to the left. 5. The myocardium is a major portion of the heart consisting mostly of cardiac muscle; its muscle fibers are branched and tightly joined together. 6. The heart lies within the pericardium, a sac that secretes a lubricating fluid. 7. The endocardium lines the inner surface of the heart; it consists of connective tissue and endothelial tissue. 77 8. An internal wall called the septum separates the heart into right and left halves. 9. The heart has two upper, thin-walled atria and two lower, thick-walled ventricles. 10. Heart valves direct the flow of blood and prevent any backward movement. a. Valves are supported by strong fibrous tendons (chordae tendineae) which support the valves and prevent them from inverting when the heart contracts. b. Atrioventricular valves between the atria and ventricles prevent any back flow from the ventricle to the atrium. c. The right atrioventricular (tricuspid) valve on right side of the heart consists of three cusps or flaps. d. The left atrioventricular (bicuspid or mitral) valve on left side consists of two cusps or flaps. e. Semilunar valves are located between the ventricles and their attached vessels. 1) The pulmonary semilunar valve lies between the right ventricle and the pulmonary trunk. 2) The aortic semilunar valve lies between the left ventricle and the aorta. B. Path of Blood Through the Heart 1. The route of blood through the heart is as follows: a. Oxygen-poor blood enters the right atrium from both the superior vena cava and the inferior vena cava. b. The right atrium sends blood through the right atrioventricular (tricuspid) valve to the right ventricle. c. The right ventricle sends blood through the pulmonary semilunar valve into the pulmonary trunk and arteries to the lungs. d. Oxygen-rich blood returns from the lungs through pulmonary veins and is delivered to the left atrium. e. The left atrium sends blood through the left atrioventricular (bicuspid or mitral) valve to the left ventricle. f. The left ventricle sends blood through the aortic semilunar valve into the aorta and on to the body proper. 2. The heart is therefore a double pump serving the lungs and body circulations simultaneously; O2-poor blood and O2-rich blood never mix. 3. Since the left ventricle has the harder job of pumping blood throughout the body, its walls are thicker; accordingly, blood pressure is greatest in the aorta. 4. Blood pressure decreases as the cross-sectional area of the arteries and arterioles increases. C. The Heartbeat 1. The human heart contracts (beats) about 70 times a minute (2.5 billion times in a lifetime); each heartbeat lasts about 0.85 seconds. 2. The heartbeat or cardiac cycle consists of phases. 3. The atria contract first while the ventricles relax (0.15 sec.), then the ventricles contract while atria relax (0.30 sec.), and then all chambers rest (0.40 sec.). 4. Systole refers to the contraction of heart chambers and diastole refers to the relaxation of the heart chambers. 5. The heart is in diastole about 50% of the time. 6. The short systole of the atria is needed only to send blood into the ventricles. 7. When the term “systole” is used alone, it refers to the left ventricle systole; the volume of blood that the left ventricle pumps per minute into the systemic circuit is called the cardiac output. 8. When the heart beats, the familiar lub-dub sound is heard as the valves of the heart close. a. Lub is caused by the vibrations of the heart when the atrioventricular valves close. b. Dub is heard when the vibrations occur due to the closing of semilunar valves. 9. The pulse is a wave effect that passes down the walls of arterial blood vessels when the aorta expands and then recoils following ventricular systole. 10. Since there is one arterial pulse per ventricular systole, the arterial pulse rate can be used to determine the heart rate. 11. Rhythmic contraction of the heart is due to the cardiac conduction system. a. The sinoatrial (SA) node is the “pacemaker” found in the upper dorsal wall of the right 78 atrium; it initiates the heartbeat by sending out an excitatory impulse every 0.85 seconds to cause the atria to contract. b. The atrioventricular (AV) node is found in the base of the right atrium very near the septum; when stimulated by impulses from the SA node, it sends out impulses through the septum to cause the ventricles to contract. c. Although the beat of the heart is intrinsic, it is regulated by the nervous system which can increase or decrease the heartbeat rate. d. The SA node is called the cardiac pacemaker because it usually keeps the heartbeat regular. 12. An electrocardiogram (ECG) is a recording of the electrical changes that occur in the myocardium during a cardiac cycle; it is used as a diagnostic tool to identify abnormal cardiac function. 13. Normal Cardiac Cycle a. The P wave represents excitation and occurs just before atrial contraction. b. The QRS complex signals that the ventricles are about to contract. c. The electrical changes that occur as the ventricular muscle fibers recover produce the T wave. 14. Ventricular fibrillation is uncoordinated contraction of the ventricles; with the application of a strong electric current, the SA node may reestablish a coordinated beat. D. Vascular Pathways The human cardiovascular system has two major circular pathways. 1. The Pulmonary Circuit a. The pulmonary circuit circulates blood to the lungs. b. Oxygen-poor blood from the body collects in the right ventricle, which pumps it to pulmonary trunk. c. The pulmonary trunk divides into right and left pulmonary arteries to carry blood to each lung. d. In the lungs, carbon dioxide (CO2) is unloaded and O2 is picked up by blood. e. Oxygen-rich blood from the lungs is returned through pulmonary veins to the left atrium. 2. The Systemic Circuit a. The aorta and vena cavae are main pathways for blood in the systemic circuit. b. Transport of oxygenated blood moves from the left ventricle through the aorta out to all tissues. c. Deoxygenated blood returns from all tissues via the vena cavae. d. In a systemic circuit, arteries contain bright red oxygen-rich blood; the veins contain dull red oxygen-poor blood that appears blue when viewed through the skin. 3. The coronary arteries serve the heart muscle itself. a. Coronary arteries originate from the base of the aorta just above the aortic semilunar valve. b. Coronary arteries lie on the external surface of the heart; they branch into arterioles and capillaries. c. Capillary beds enter the venules that join to form the cardiac veins. d. Coronary veins collect oxygen-poor blood from the capillaries and empty it into the right atrium. 4. The portal system is a pathway of blood flow that begins and ends in capillaries. a. The hepatic portal vein transports blood from capillaries in the small intestinal villi to capillaries in the liver. b. The hepatic vein leaves the liver and enters the inferior vena cava. c. In the liver, substances absorbed by the intestine are modified, toxins and bacteria are removed, and the normal composition of blood is monitored. E. Blood Pressure 1. Systolic pressure results from blood being forced into the arteries during ventricular systole. 2. Diastolic pressure is the pressure in arteries during ventricular diastole. 3. Human blood pressure is measured as the force pushing against the wall of the brachial artery of the upper arm. a. Blood pressure is measured by a sphygmomanometer which has a pressure cuff. 79 b. 4. 5. 6. 7. 8. 9. Clinical blood pressure measures pressures produced by contraction and relaxation of the left ventricle. c. Blood pressure is stated in millimeters of mercury (e.g., 120/80 mm Hg) for systolic/diastolic. As blood flows from the aorta into arteries and arterioles, the blood pressure falls. The difference in pressure between systolic and diastolic pressures gradually diminishes. Capillaries have a slow, even blood flow due to the high total cross-sectional area. Blood pressure in the veins is low and cannot move blood back to heart, especially from the limbs. Skeletal muscle contraction on the walls of veins with valves, preventing backflow of blood, is responsible for the flow of blood in veins. Varicose veins are abnormal dilations that develop when the valves become weak and ineffective. 22.4 Cardiovascular Disorders 1. Cardiovascular disease (CVD) is the leading cause of untimely death in Western countries. 2. The risk of CVD can be reduced by following guidelines for a heart-healthy life-style. A. Hypertension 1. An estimated 20% of Americans suffer from hypertension (high blood pressure). 2. Women have this condition if their blood pressure is significantly higher than 160/95; men under the age of 45 if over 130/90, and beyond the age of 45 if above 140/95. 3. The diastolic pressure is what is emphasized when medical treatment is considered. 4. Hypertension may not be detected until a stroke or heart attack occurs; for this reason, hypertension is often called a silent killer. 80 5. Two genes are involved in hypertension for some individuals. a. One gene codes for angiotensinogen, a plasma protein converted to a vasoconstrictor by the product of a second gene. b. Persons with this form of hypertension may one day be cured by gene therapy. B. Atherosclerosis 1. Hypertension is seen in individuals with atherosclerosis (formerly called arteriosclerosis). 2. Soft masses of fatty materials, mostly cholesterol, accumulate beneath the inner linings of arteries. 3. As this plaque accumulates, it protrudes into the vessel and interferes with blood flow. 4. Atherosclerosis develops in early adulthood but the symptoms may not appear until age 50 or older. 5. Plaque can cause a blood clot to form on irregular arterial walls. 6. As long as a clot remains stationary, it is a thrombus. 7. If a clot dislodges, it is an embolus, a blood clot that moves in the blood. 8. In some families, atherosclerosis is inherited as familial hypercholesterolemia. C. Stroke and Heart Attack 1. Stroke, heart attack, and aneurysm are associated with hypertension and atherosclerosis. 2. A stroke can result in paralysis or death; a small cranial arteriole bursts or is blocked by an embolus. a. A stroke is also called a cardiovascular accident (CVA). b. Whether paralysis or death occurs depends on the extent of the portion of the brain that lacks O2. c. Warning symptoms that foretell stroke include: numbness in hands or face, difficulty speaking, blindness in one eye, etc. 3. A myocardial infarction (MI) is also called heart attack. a. This occurs when a portion of heart muscle dies due to a lack of O 2; this may be caused by a thromboembolism blocking a coronary artery. b. A partially blocked coronary artery causes angina pectoris causing pains or a flash of burning. c. Nitroglycerin and related drugs dilate the blood vessels and relieve pain. 22.5 Blood, a Transport Medium The blood of mammals has two components: plasma and formed elements (cells and platelets). 1. Plasma contains water and many types of molecules, including nutrients, wastes, salts, and proteins. 2. Salts and proteins buffer the blood. a. They effectively keep the blood pH near 7.4. b. They maintain the blood osmotic pressure so water has a tendency to enter capillaries. 3. Some plasma proteins are involved in blood clotting. 4. Some plasma proteins assist in transporting large organic molecules in the blood. a. Lipoproteins that transport cholesterol are globulins. b. Albumin, a common plasma protein, transports bilirubin, a breakdown product of hemoglobin. A. Formed Elements 1. Formed elements are of three types: red blood cells (RBCs), white blood cells (WBCs), and platelets. 2. Red Blood Cells a. Red blood cells (erythrocytes) are small biconcave disks. b. When mature, RBCs lack a nucleus and contain hemoglobin. c. There are 6 million RBCs per mm3 of whole blood. d. Each RBC contains about 250 million hemoglobin molecules. 1) Hemoglobin contains four globin protein chains, each with an iron-containing heme group. 2) The iron atom of a heme group loosely binds with an O 2 molecule; thus, blood carries oxygen. 3) Anemia is either a lack of enough RBC or insufficient hemoglobin; an individual 81 e. f. g. h. suffers from a tired, run-down feeling. RBCs are manufactured in the red bone marrow of the skull, ribs, vertebrae, and the ends of long bones. The growth factor erythropoietin is produced when an enzyme from the kidneys acts on a precursor made by the liver and stimulates production of red blood cells; as a drug it helps people with anemia. Before being released from bone marrow, the RBCs lose their nucleus and synthesize hemoglobin. Red blood cells have a life span of about 120 days; then they are destroyed chiefly in the liver and spleen. 82 i. 3. 4. 5. When the RBCs are destroyed, the hemoglobin is released; the iron is recovered and returned to the bone marrow where it is reused. j. The heme portions undergo chemical degradation and are excreted by the liver as bile pigments; it colors the feces. White Blood Cells a. White blood cells (leukocytes) differ from RBCs in being larger and in having a nucleus. b. WBCs lack hemoglobin and appear translucent without staining. c. Granular leukocytes contain conspicuous granules in their cytoplasm and have a lobed nucleus. 1) Neutrophils have granules that stain slightly pink; they are amoeboid, spherical cells that readily squeeze through capillary walls and phagocytize foreign material. 2) Eosinophils have granules that take up the red dye eosin. 3) Basophils have granules that take up a basic dye, staining them deep blue. d. A newly discovered stem cell growth factor (SGF) increases the production of all WBCs, which helps patients with low immunity. e. Agranular leukocytes lack granules in their cytoplasm and have a circular or indented nucleus. 1) Monocytes are amoeboid and able to enter tissues where they transform into macrophages. 2) Macrophages release white blood cell growth factors that increase the number of leukocytes. 3) Pus is a thick, yellowish fluid that contains a large proportion of dead WBCs that have fought infection. 4) Lymphocytes play a key role in fighting infection and include two types. a) T cells are lymphocytes that directly attack virus-infected cells. b) B cells can be stimulated to produce one type of antibody specific for one type of antigen. 5) An antigen is any substance stimulating production of antibodies; antigen is foreign to the body. 6) Antibodies combine with antigens to promote their being phagocytized by a macrophage. 7) A person is actively immune when many B cells produce a specific antibody for an infection. Platelets a. Platelets (thrombocytes) result from fragmented giant cells (megakaryocytes) in the bone marrow. b. 200 billion platelets are produced a day; blood contains 150,000–300,000 platelets per mm3. c. Platelets are involved in blood clotting, or coagulation. d. At least 12 clotting factors in the blood participate in blood clotting. e. Hemophilia is an inherited disorder where the liver is unable to produce one of the clotting factors. f. In hemophilia, minor bumps can cause internal bleeding; bleeding into the brain causes death. g. Vitamin K is necessary to produce prothrombin; deficiency of vitamin K causes hemorrhagic disorders. Blood Clotting a. When a blood vessel is damaged, platelets clump at the site of the puncture and partially seal the leak. b. The platelets and damaged tissue cells release a clotting factor called prothrombin activator. c. With calcium ions, prothrombin activator catalyzes a reaction converting prothrombin to thrombin. d. Thrombin acts as an enzyme to sever two amino acid chains from each fibrinogen molecule. 83 e. f. g. h. These activated fragments join end-to-end forming long threads of fibrin. Fibrin threads wind around the platelet plug and provide a framework for a clot. RBCs are trapped within the fibrin threads, making the clot appear red. When blood vessel repair is initiated, plasmin destroys the fibrin network and restores plasma fluidity. i. When clotting occurs in a test tube, a fluid serum collects above a clot; it has the same composition as plasma except fibrinogen. B. Capillary Exchange 1. Two forces control the movement of fluid through the capillary walls. a. Osmotic pressure tends to cause water to move from tissue fluid to the blood. b. Blood pressure tends to cause water to move from the blood to tissues. 84 c. 2. 3. 4. 5. 6. 7. 8. At the arterial end of a capillary, blood pressure is higher than osmotic pressure: water exits and moves into tissues. d. Along the capillary, O2 and nutrients diffuse out into the tissue fluid, while CO2 and other metabolic wastes diffuse into the capillaries from the tissue fluid. Midway along a capillary, there is no net movement of water. The tissue fluid is intercellular fluid that surrounds the cells; the circulatory system exchanges materials with this fluid. The exchange between the blood and tissue fluid occurs by diffusion through the one-cell-thick capillary walls. a. At the venule end, osmotic pressure is higher than blood pressure and water moves back into the blood. b. Almost the same amount of fluid that left the capillary returns to it; there is always some excess tissue fluid collected by the lymphatic capillaries. The tissue fluid within lymphatic vessels is lymph. Lymph returns to the systemic venous blood when lymphatic vessels enter the subclavian veins in the shoulder. Not all capillary beds are open at the same time; precapillary sphincters shunt blood along various pathways. Through capillary dilation and constriction, blood also distributes heat to body parts and conserves heat when cold. Critical Thinking Question 1. The kidney receives about one-fifth of the blood flow; this translates into a red blood cell passing through the kidney once for every four times it makes a circuit through the rest of the body. How does this compare to blood flow through the lungs? Question 2. The percentage of North American Indians with type B blood is only 1%, and virtually none has type AB. Yet, anthropologists believe they originated from northern Asia, and the percentages of Americans with types B and AB are 35% and 10% respectively. What evolutionary mechanism could account for this? Question 3. The lung of a fetus is not yet functioning to absorb oxygen-rich blood from air; the placental umbilical cord functions to deliver this through the abdomen to the fetus’s main blood vessels. Before birth, the fetal heart therefore has an opening, called the foramen ovale, in the septum between the right and left ventricle. If this does not close by birth, the infant may require heart surgery. (1) Why does this opening pose a problem for a newborn only after birth, and what symptoms would you expect? (2) If this is such a potential danger, why hasn’t evolution eliminated the opening completely? Question 4. The umbilical cord of the fetus is part of the fetal blood system and leads to the placental interface where oxygen and carbon dioxide diffuse across, as well as food molecules and metabolic wastes. How are the arteries and veins in the umbilical cord similar to the pulmonary circuit? Question 5. “Blood doping” occurs when an athlete or horse owner removes blood, stores it, then returns it after the body has had time to restore normal blood levels. Why is this sometimes done and how would this be diagnosed? 85 CHAPTER 24 DIGESTIVE SYSTEMS AND NUTRITION Chapter Outline 24.1 Digestive Tracts 1. Most animals need to digest food into small molecules that can cross plasma membranes. 2. Digestion provides the energy needed to carry out routine metabolic activities and maintain homeostasis. 3. 4. 5. 6. 7. 8. 9. A. Mouth The digestive tract ingests food, breaks down food into small molecules that can cross plasma membranes, absorbs these nutrient molecules, and eliminates nondigestible remains. The human digestive tract is a complete tube-within-a-tube system. Each part of the digestive system has a specific function. Food is never found within the accessory glands, only within the tract itself. The digestion of food in humans is an extracellular process. Enzymes are secreted into the digestive tract by nearby glands which never contain food themselves. Nutritional homeostasis is under hormonal and nervous regulations. Food is chewed in the mouth (oral cavity) and mixed with saliva; the mouth is the beginning of the digestive tract. 1. Three pairs of salivary glands secrete saliva by way of ducts into the mouth. 2. Salivary contains amylase, protease and lipase. 3. Food is manipulated by a muscular tongue containing both touch and pressure receptors. 4. Taste buds are located primarily on the tongue but also on the surface of the mouth; these chemical receptors are stimulated by the chemical composition of food. 5. Food is chewed and mixed with saliva to form a bolus in preparation for swallowing. B. The Pharynx and the Esophagus 1. The digestive and respiratory passages come together in the pharynx, and then separate. a. During swallowing, the pathway of air to the lungs could be blocked if food entered the trachea. b. The epiglottis covers the opening into the trachea as muscles move a bolus of food through the pharynx into the esophagus. 2. The esophagus is a muscular tube that moves swallowed food to the stomach by peristalsis, a rhythmical contraction that moves the contents along in tubular organs. C. Stomach 1. The stomach stores liters of partially digested food, freeing humans from continual eating. 2. Dr. William Beaumont revealed much of the stomach’s functions in the mid-1800s. a. Alexis St. Martin had an opening (fistula) into the stomach, received from a gunshot, through which Dr. Beaumont could observe stomach activity. b. Beaumont collected the gastric juice produced by cells of gastric glands. c. Walls of the stomach contract vigorously and mix food with juices secreted when the food enters. d. Beaumont found that gastric juice contains hydrochloric acid and another digestive substance, pepsin. 3. Hydrochloric acid (HCl) lowers pH of the gastric contents to about 2. a. The epithelial lining of the stomach has millions of gastric pits leading to gastric glands. b. This acid kills most bacteria and other microorganisms. c. The low pH also stops the activity of salivary amylase and promotes the activity of 86 pepsin, an enzyme that digests large proteins to smaller peptides. 87 4. 5. A thick layer of mucus protects the wall of the stomach from the HCl and pepsin. Ulcers develop when the lining is exposed to digestive action; recent research indicates this is usually due to infection by Helicobacter pylori bacteria. 6. Stomach contents, a thick, soupy mixture, is called chyme. 7. At the base of the stomach is a narrow opening controlled by a sphincter (a circular muscle valve). a. When the sphincter relaxes, chyme enters the first part of the small intestine, called the duodenum; a neural reflex causes the sphincter to contract, closing off the opening. b. The sphincter relaxes and allows more chyme to enter the duodenum. c. The slow, rhythmic pace with which chyme exits the stomach allows for thorough digestion. D. The Small Intestine 1. The human small intestine is a coiled muscular tube about three meters long. 2. As chyme enters the duodenum, proteins and carbohydrates are partly digested but no fat digestion occurs. 3. Additional digestion is aided by secretions from the liver and the pancreas (below). Bile is a secretion of the liver temporarily stored in the gallbladder before being sent to duodenum; bile emulsifies fat (allows fat droplets to disperse in water). 4. The lining of the small intestine has ridges and furrows; these surfaces are covered by villi (sing. villus); the small intestine is specialized for absorption by the huge number of villi that line the intestinal wall. a. Villi are fingerlike projections; their surface cells are covered by microvilli. b. Microvilli are minute projections, called the “brush border,” on the surface of the cells of the intestinal villi. c. Ridges, furrows, villi, and microvilli greatly increase the effective surface area of the small intestine. 5. Each villus contains blood vessels and a lymphatic capillary, called a lacteal.. A lacteal aids in the absorption of fats. 6. Sugars and amino acids enter villi cells and are absorbed into bloodstream. 7. Glycerol and fatty acids enter villi cells; reassembled into fat molecules, they move into lacteals. 8. After nutrients are absorbed, they are eventually carried throughout the body by the bloodstream. E. Large Intestine 1. The large intestine has four parts: the cecum, colon, rectum, and anal canal. 2. It is larger in diameter but shorter in length than the small intestine and is the region following the small intestine. 3. Appendix a. The appendix is a fingerlike projection extending from the cecum, a blind sac at the junction of the small and large intestine. b. It may play a role in fighting infections. c. If an infected appendix bursts, it results in general abdominal infection , called peritonitis. 4. The colon is subdivided into the ascending, transverse, descending, and sigmoidal colon. 5. About 1.5 liters of water enter the digestive tract daily from ingestion and another 8.5 liters enter from various secretions. a. About 95% of this total liquid is reabsorbed by the small intestine; most of the remainder is absorbed by cells of the colon. b. If the water is not reabsorbed, it causes diarrhea which can cause a serious dehydration and ion loss. 6. In addition to water, the large intestine absorbs salts and some vitamins, including the vitamin K produced by intestinal bacteria. 7. The large intestine terminates at the anus, the opening of the anal canal. 8. A low-fat, high-fiber diet promotes regularity and may provide protection against mutagenic agents. F. Three Accessory Organs 88 1. The Pancreas a. The pancreas lies deep within the abdominal cavity, just below the stomach, and rests on the posterior abdominal wall. b. It is an elongated and somewhat flattened organ. c. As an endocrine gland, it secretes glucagon and insulin hormone into the bloodstream. d. As an exocrine gland, it secretes pancreatic juice. 1) Pancreatic juice contains sodium bicarbonate that neutralizes acidic chyme. 2) Pancreatic enzymes digest carbohydrates, fats and proteins. 2. The Liver a. The liver, the largest gland in the body, fills the top of the abdominal cavity, just under the diaphragm. b. The liver has numerous functions: 1) It detoxifies blood by removing and metabolizing poisonous substances. 2) It makes plasma proteins including albumin and fibrinogen. 3) It destroys old red blood cells and converts hemoglobin to bilirubin and biliverdin in bile. 4) It produces bile stored in the gallbladder before it enters the duodenum to emulsify fats. 5) It stores glucose as glycogen and breaks down glycogen to maintain a constant blood glucose concentration. 6) The liver produces urea from amino groups and ammonia. c. Blood vessels from both the large and small intestines lead to the liver as the hepatic portal vein. d. The liver maintains the blood glucose level at 0.1% by removing glucose from the hepatic portal vein to store as glycogen; when needed, glycogen is broken down and glucose re-enters the hepatic vein. e. Amino acids can be converted to glucose but deamination (removal of amino groups) must occur. f. By a complex metabolic pathway, the liver converts amino groups to urea, the nitrogenous breakdown product of amino acids. g. Urea is the most common human nitrogenous waste; it is transported by the blood to the kidneys. 3. Liver Disorders a. Jaundice is a symptom involving a yellowish skin due to a large amount of bilirubin in blood. 1) Jaundice can also result from hepatitis, inflammation of the liver. b. Viral hepatitis is a viral liver infection. 1) Hepatitis A results from eating contaminated food. 2) Hepatitis B and C are spread by blood transfusions, kidney dialysis, and unsterile needle use. 3) All three can be caused from sexual contact. c. Cirrhosis is a chronic disease where the liver tissue is replaced by fatty tissue and then scar tissue; alcoholics provide too much alcohol for the liver to break down. 4. The Gallbladder a. The gallbladder is a pear-shaped, muscular sac attached to the surface of the liver. b. Bile, produced in the liver, is stored in the gallbladder. c. When needed, bile leaves the gallbladder and goes into the duodenum via the common bile duct. 24.2 Digestive Enzymes 1. Salivary amylase is the enzyme that begins starch digestion; maltose is the common end product. Maltose cannot be absorbed in the small intestine; additional digestive action breaks the maltose into glucose, which can be absorbed. 2. Protein digestion begins in the stomach. Pepsinogen is converted to pepsin when exposed to the HCl in the stomach; pepsin breaks proteins into smaller peptides. 89 3. Starch, proteins, nucleic acids, and fats are enzymatically broken down in the small intestine. a. Pancreatic amylase digests starch to maltose. b. Trypsin, also a pancreatic enzyme, digests protein to peptides. c. Peptidases and maltase, produced by the small intestine, complete the digestion of protein to amino acids and starch to glucose, respectively. d. Lipase, another pancreatic enzyme, digests fat droplets to glycerol and fatty acids. 24.3 Nutrition 1. Nutrients in the diet provide energy, promote growth and development, and regulate cellular metabolism. 2. Three eating disorders: a. Obesity (body weight >19% above ideal weight for a person’s height) can lead to diabetes, cardiovascular (CV) problems, and perhaps cancer. b. In bulimia nervosa, individuals binge eat then purge themselves with laxitives or self-induced vomiting. c. Individuals with anorexia nervosa have a fear of weight gain; they literally starve themselves to appear thin—this leads to bone density decrease, CV problems, and possibly a general “shut down” of the body. A. Carbohydrates 1. Complex carbohydrates (e.g., whole grain cereals, breads, etc.) are recommended because they are digested to sugars and contain fiber. a. Insoluble fiber (e.g., as found in wheat bran) has a laxative effect and may guard against colon cancer. b. Soluble fiber (e.g., as found in oat bran) combines with bile acids and cholesterol in the intestine and prevents their absorption. 2. Simple sugars (e.g., candy) and the sugars obtained from the starch in potatoes and white bread, have a high glycemic index (GI) because the blood glucose response to these foods is high. B. Proteins 1. Of the 20 different amino acids required for protein synthesis, 8 (9 in children) cannot be synthesized by the body and are thus termed essential amino acids. 2. Some foods do not provide all the essential amino acids—vegetarians should combine two or more plant products to acquire all the essential amino acids. 3. A high-protein diet can harm the body. a. Dehydration can occur. b. Calcium loss in the urine can occur, leading to kidney stones. C. Lipids 1. Saturated fats (i.e., solid at room temperature) usually have an animal origin. 2. Oils contain unsaturated fatty acids, which do not promote CV disease; omega-3 fatty acids, found in some cold-water fishes and in flax seed oil, are believed to prevent heart disease. 3. Fats That Cause Disease a. CV disease is often due to arteries being blocked by plaque, which contains saturated fats and cholesterol. 90 b. Trans fatty acids are more likely to cause CV disease than saturated fats—any packaged goods that contain partially hydrogenated vegetable oils (“shortening”) will likely contain trans fat. D. Vitamins 1. Vitamins are essential organic compounds the body cannot make but still requires for metabolic activities. 2. Many vitamins are portions of coenzymes: e.g., niacin is part of NAD+ and riboflavin is a part of FAD. 3. Coenzymes are needed in small amounts because they are used repeatedly. 4. Vitamin A is not a coenzyme but a precursor for the visual pigment that prevents night blindness. 5. Lack of vitamins results in vitamin deficiencies. 6. The 13 vitamins are divided into those that are fat soluble and those that are water soluble. E. Antioxidants 1. Cell metabolism generates free radicals, unstable molecules with an extra electron; O 2- is a common free radical. 2. Free radicals stabilize by eventually donating electrons to another molecule; this damages cellular molecules. 3. Free radicals damage DNA, proteins, and other molecules by donating an electron; this may cause cancer or plaque in arteries. 4. Vitamins C, E, and A—abundant in fruits and vegetables—are antioxidants that defend against free radicals. 5. Supplements do not replace fruits and vegetables that also contain many other beneficial compounds. F. Minerals 1. Humans require certain minerals (e.g., calcium, phosphorus) in amounts of over 100 mg per day. a. They are constituents of cells and body fluids and structural components of tissues. b. Calcium is needed to build bones and teeth and for nerve conduction and muscle contraction. 2. Certain other minerals are elements (e.g., zinc, iron) recommended in amounts less than 20 mg per day. a. These microminerals are more likely to have very specific functions. b. Iron is needed to produce hemoglobin; adult females need more due to loss of menstrual blood. c. Iodine is used to produce thyroxin, a hormone of the thyroid glands. d. Minute amounts of molybdenum, selenium, chromium, nickel, vanadium, silicon, and arsenic are essential. e. Some individuals may not receive enough calcium; stress can cause a magnesium deficiency, and a vegetarian diet may be short on zinc. G. Calcium 1. Calcium supplements counteract the osteoporosis that afflicts 25% of older men and 50% of older women. 2. Porous bones break easily due to lack of calcium. 3. After menopause, bone-eating cells called osteoclasts are more active than bone-forming osteoblasts. 4. Calcium supplements have been shown to slow bone loss in the elderly. 5. Intake of 1,000–1,500 mg calcium/day is recommended; therefore supplemental calcium is usually necessary. 6. Exercise is also effective in building bone mass. H. Sodium 1. The recommended daily intake of sodium is 400–3,300 mg; the average American intake is 4,000–4,700 mg. 2. A high sodium intake has been linked to hypertension in some people. 3. One third of our sodium intake is found naturally in foods; another third is added in 91 4. processing. We add one-third of our salt intake in cooking or as table salt. Critical Thinking Question 1. Sometimes a section of intestine must be removed when it is found to be cancerous. Which would most adversely affect the functioning of the total digestive system: the loss of one foot of duodenum, one foot of the lower small intestine, or one foot of the large intestine? Why? Question 2. Although we have developed artificial kidneys and hearts, there really is no machine that can function as an artificial liver. Why is it so difficult to replicate all of the functions of the liver? Question 3. There are a few populations of people on the globe who have a long tradition of many more individuals living to great old age (over 100). When emigrants from these regions live in modern settings with ample diets, their survivorship is lower and near average levels. The one common factor appears to be a much lower caloric intake, perhaps 70% of the average daily allowance considered “normal.” Discuss this in the light of “antioxidants.” Question 4. If the food product is appetizing to a person, why is it unwise to focus on a diet of just this one food item, even if it is high in protein like soybeans? 92 Chapter 25 Neurons and Nervous Systems Chapter Outline 25.1 The Human Nervous System Three specific functions of the nervous system are to: 1. 2. 3. 4. a. receive sensory input, b. perform integration, and c. generate motor output to muscles and glands. The central nervous system (CNS) consists of the brain (in the skull) and the spinal cord (in the vertebral column). The peripheral nervous system (PNS) lies outside the CNS and contains the cranial and spinal nerves. The PNS is divided into the somatic and autonomic systems. a. The somatic system controls the skeletal muscles. b. The autonomic system controls the smooth muscles, cardiac muscles, and glands. The CNS and PNS of the human nervous system are connected and work together to perform the functions of a nervous system. 25.2 Nervous Tissue Nervous tissue is made up of neurons (nerve cells) and neuroglia (which support and nourishe the neurons). A. Neurons and Neuroglia 1. Neurons vary in appearance, depending on their function and location, but they all have three parts. a. b. c. The cell body contains the nucleus and other organelles. Dendrites receive information and conduct impulses toward the cell body. A Single axon conducts impulses away from the cell body to stimulate or inhibit a neuron, muscle, or gland. 1) A long axon is called a nerve fiber. 2) The long axons are covered by a white myelin sheath. 2. Types of Neurons a. Motor (efferent) neurons have many dendrites and a single axon; they conduct impulses from the CNS to muscles or glands. b. Sensory (afferent) neurons are unipolar; they conduct impulses from the periphery toward the CNS. 1) The process that extends from the cell body divides into two processes, one going to the CNS and one to periphery. c. Interneurons (association neurons) are multipolar 1) They have highly-branched dendrites within the CNS. 2) Interneurons convey messages between the various parts of the CNS. 3) They form complex brain pathways accounting for thinking, memory, language, etc. B. Transmission of the Nerve Impulses 1. 2. 3. 4. In 1786, Luigi Galvani discovered that a nerve can be stimulated by an electric current. An impulse is too slow to be due to simply an electric current in an axon. Julius Bernstein (early 1900s) proposed that the nerve impulse is the movement of unequally distributed ions on either side of an axonal membrane, the plasma membrane of an axon. A. L. Hodgkin and A. F. Huxley later confirmed this theory. a. They and other researchers inserted a tiny electrode into the giant axon of a squid. b. The electrode was attached to a voltmeter and an oscilloscope to trace a change in voltage over time. 93 c. The voltage measured the difference in the electrical potential between the inside and outside of the membrane. d. An oscilloscope indicated any changes in polarity. 5. Resting Potential a. When an axon is not conducting an impulse, an oscilloscope records a membrane potential equal to negative 70 mV, indicating that the inside of the neuron is more negative than the outside. b. This is the resting potential because the axon is not conducting an impulse. c. This polarity is due to the difference in electrical charge on either side of the axomembrane. 1) The inside of the plasma membrane is more negatively charged than the outside. 2) Although there is a higher concentration of K + ions inside the axon, there is a much higher concentration of Na+ ions outside the axon. 3) The plasma membrane is more permeable to K+ ions, so this gradient is less and the K+ ion potential is less. 4) The sodium-potassium pump maintains this unequal distribution of Na + and K+ ions. d. The sodium-potassium (Na+-K+) pump is an active transport system that moves Na + ions out and K+ ions into the axon. e. The pump is always working because the membrane is permeable to these ions and they tend to diffuse toward the lesser concentration. f. Since the plasma membrane is more permeable to potassium ions than to sodium ions, there are always more positive ions outside; this accounts for some polarity. g. The large negatively charged proteins in the cytoplasm of the axon also contribute to the resting potential of – 70 mV. 6. Action Potential a. When an axon conducts a nerve impulse, the rapid change in the membrane potential is the action potential. b. Protein-lined channels in the axomembrane open to allow either sodium or potassium ions to pass; these are sodium and potassium gated ion channels. c. The action potential is generated only after the occurrence of a threshold value. d. The oscilloscope goes from –70 mV to +40 mV in a depolarization phase, indicating the cytoplasm is now more positive than the tissue fluid. e. The trace returns to –70 mV again in the repolarization phase, indicating the inside of the axon is negative again. 7. Propagation of Action Potentials a. If an axon is unmyelinated, an action potential stimulates an adjacent axomembrane to produce an action potential. b. In myelinated fibers, the action potential at one neurofibril node causes action potential at the next node. 1) The myelinated sheath has neurofibril nodes, gaps where one neurolemmocyte ends and the next begins. 2) The action potential “leaps” from one neurofibril node to another—this is called saltatory conduction. 3) Saltatory conduction may reach rates of over 100 meters/second, compared to 1 meter/second without it. c. As each impulse passes, the membrane undergoes a short refractory period before it can open the sodium gates again. d. The conduction of a nerve impulse is an all-or-nothing event. e. This ensures a one-way direction to the impulse; during a refractory period, sodium gates cannot open. C. Transmission Across a Synapse 1. The minute space between the axon bulb and the cell body of the next neuron is the synapse. 2. A synapse consists of a presynaptic membrane, a synaptic cleft, and the postsynaptic membrane. a. Synaptic vesicles store neurotransmitters that diffuse across the synapse. b. When the action potential arrives at the presynaptic axon bulb, synaptic vesicles merge 94 with the presynaptic membrane. When vesicles merge with the membrane, neurotransmitters are discharged into the synaptic cleft. d. The neurotransmitter molecules diffuse across the synaptic cleft to the postsynaptic membrane where they bind with specific receptors. e. The type of neurotransmitter and/or receptor determines if the response is excitation or inhibition. f. Excitatory neurotransmitters use gated ion channels and are fast acting. g. Other neurotransmitters affect the metabolism of the postsynaptic cells and are slower. 3. Neurotransmitters and Neuromodulators a. At least 100 different neurotransmitters have been identified. b. Acetylcholine (ACh) and norepinephrine (NE), dopamine, and serotonin are present in both the CNS and the PNS. 1) ACh can have either an excitatory or an inhibitory effect, depending on the tissue. 2) NE is important to dreaming, waking, and mood. 3) Dopamine is involved in emotions, learning, and attention. 4) Serotonin is involved in thermoregulation, emotions, and perception. c. Once a neurotransmitter is released into a synaptic cleft, it initiates a response and is then removed from the cleft. d. In some synapses, the postsynaptic membrane contains enzymes that rapidly inactivate the neurotransmitter. e. Acetylcholinesterase (AChe) breaks down acetylcholine. f. In other synapses, the presynaptic membrane reabsorbs the neurotransmitter for repackaging in synaptic vesicles or for molecular breakdown. g. The short existence of neurotransmitters in a synapse prevents continuous stimulation (or inhibition) of postsynaptic membranes. h. Many drugs that affect the nervous system act by interfering with or potentiating the action of neurotransmitters. i. Neuromodulators are molecules that block the release of a neurotransmitter or modify a neuron’s response to one. 1) Substance P is released by sensory neurons when pain is present; endorphins block the release of substance P and therefore act as natural painkillers. D. Synaptic Integration 1. A neuron has many dendrites and may have one to ten thousand synapses with other neurons. 2. A neuron receives many excitatory and inhibitory signals. 3. Excitatory neurotransmitters produce a potential change (signal) that drives the neuron closer to an action potential; inhibitory signals produce a signal that drives the neuron further from an action potential. 4. Thus excitatory signals have a depolarizing effect and inhibitory signals have a hyperpolarizing effect. 5. Integration is the summing up of excitatory and inhibitory signals. a. If a neuron receives many excitatory signals, or at a rapid rate from one synapse, the axon will probably transmit a nerve impulse. b. If both positive and inhibitory signals are received, the summing may prohibit the axon from firing. c. 25.3 Central Nervous System: Brain and Spinal Cord 1. 2. 3. 4. 5. The central nervous system (spinal cord and brain) is where sensory impulses are received and motor control is initiated. Both the brain and the spinal cord are protected by bone. Both are wrapped in three connective tissue coverings called meninges; meningitis is a disease caused by many different bacteria or viruses that invade the meninges. The spaces between the meninges are filled with cerebrospinal fluid to cushion and protect the CNS. The cerebrospinal fluid is contained in the central canal of the spinal cord and within the ventricles of the brain. 95 6. The ventricles are interconnecting spaces that produce and serve as reservoirs for the cerebrospinal fluid. A. The Spinal Cord 1. The spinal cord has two main functions. a. It is the center for many reflex actions. b. It provides the means of communication between the brain and the spinal nerves. 2. The spinal cord is composed of white and gray matter. a. Gray Matter 1) The unmyelinated cell bodies and short fibers give gray matter its color. 2) In a cross section, the gray area looks like a butterfly or the letter H. 3) It contains portions of sensory neurons and motor neurons; short interneurons connect them. b. White Matter 1) Myelinated long fibers of interneurons run together in tracts and give the white matter its color. 2) Tracts conduct impulses between the brain and the spinal nerves; ascending tracts are dorsal and descending tracts from the brain are ventral. 3) Tracts cross over near the brain; therefore the left side of the brain controls the right side of the body. c. If a spinal cord injury occurs in the cervical region, the condition of quadriplegia (paralysis of all four limbs) results. d. If the injury is in the thoracic region, the lower limbs may be paralyzed (paraplegia). B. The Brain 1. The brain has four ventricles: two lateral ventricles and a third and fourth ventricle. 2. The cerebrum is associated with the two lateral ventricles, the diencephalon with the third, and the brain stem and cerebellum with the fourth. 3. The Cerebrum a. The cerebrum, also called the telencephalon, is the largest part of the brain in humans. b. It is the last center receiving sensory input and carrying out integration to command motor responses. c. The cerebrum carries out higher thought processes for learning and memory, language and speech. d. The right and left cerebral hemispheres (the two halves of the cerebrum) are connected by a bridge of nerve fibers, the corpus callosum; different functions are associated with the two hemispheres. e. The outer portion is a highly convoluted cerebral cortex consisting of gray matter containing cell bodies and short unmyelinated fibers. f. The cerebral cortex in each hemisphere contains four surface lobes: the frontal, parietal, occipital, and temporal lobes. g. Different functions are associated with each lobe. h. The cerebral cortex contains motor, sensory, and association areas. 1) The human hand takes up a large proportion of the primary motor area. 2) The ventral to the primary motor area is a premotor area that organizes motor functions before the primary area sends signals to the cerebellum. 3) The left frontal lobe has Broca’s area for our ability to speak. 4) Sensory information from the skin and skeletal muscles arrives at a primary somatosensory area. 5) The primary visual area in the occipital lobe receives information from the eyes; a visual association area associates new visual information with old information. 6) The primary auditory area in the temporal lobe receives information from our ears. 7) The primary taste area is in the parietal lobe. 8) The somatosensory association area processes and analyzes sensory information from skin and muscles. 9) A general interpretation area receives information from all of the sensory association areas and allows us to quickly integrate signals and send them to the prefrontal area for immediate response. 96 i. j. 10) The prefrontal area in the frontal lobe receives input from other association areas and reasons and plans. White Matter 1) White matter in the CNS consists of long myelinated axons organized into tracts. 2) Descending tracts from the primary motor area communicate with lower brain centers. 3) Ascending tracts from lower brain centers send sensory information up to the primary somatosensory area. 4) These tracts cross over near the brain; therefore the left side of the brain controls the right side of the body. Basal Nuclei 1) Aside from the tracts, there are masses of gray matter located deep within the white matter. 2) These basal nuclei integrate motor commands; malfunctions cause Huntingdon and Parkinson disease. 4. The Diencephalon a. The hypothalamus and thalamus are in a portion of the brain known as the diencephalon, where the third ventricle is located. b. The hypothalamus forms the floor of the third ventricle. c. The hypothalamus maintains homeostasis. 1) It is an integrating center that regulates hunger, sleep, thirst, body temperature, water balance, and blood pressure. 2) It controls the pituitary gland and thereby serves as a link between the nervous and endocrine systems. d. The thalamus consists of two masses of gray matter in the sides and roof of the third ventricle. 1) It is the last portion of the brain for sensory input before the cerebrum. 2) It is a central relay station for sensory impulses traveling up from the body or from the brain to the cerebrum. 3) Except for smell, it channels sensory impulses to specific regions of the cerebrum for interpretation. e. The pineal gland, which secretes the melatonin hormone, is in the diencephalon. 5. The Cerebellum a. The cerebellum is separated from the brain stem by the fourth ventricle. b. The cerebellum is in two portions joined by a narrow median portion. c. The cerebellum integrates impulses from higher centers to coordinate muscle actions, maintain equilibrium and muscle tone, and sustain normal posture. d. It receives information from the eyes, inner ear, muscles, etc. indicating body position, integrates the information and sends impulses to muscles maintaining balance. e. The cerebellum assists in the learning of new motor skills, as in sports or playing the piano; it may be important in judging the passage of time. 6. The Brain Stem a. The brain stem contains the medulla oblongata, pons, and midbrain. b. Besides acting as a relay station for tracts passing between the cerebrum and spinal cord or cerebellum, the midbrain has reflex centers for visual, auditory, and tactile responses. c. The pons (“bridge”) contains bundles of axons traveling between the cerebellum and rest of the CNS. 1) The pons functions with the medulla to regulate the breathing rate. 2) It has reflex centers concerned with head movements in response to visual or auditory stimuli. d. The medulla oblongata lies between the spinal cord and the pons, anterior to the cerebellum. 1) It contains vital centers for regulating heartbeat, breathing, and vasoconstriction. 2) It contains reflex centers for vomiting, coughing, sneezing, hiccuping, and swallowing. 97 3) It contains nerve tracts that ascend or descend between the spinal cord and the brain’s higher centers. 7. The Limbic System a. The limbic system is a complex network of tracts and nuclei that incorporate medial portions of cerebral lobes, subcortical nuclei, and diencephalon. b. It blends higher mental functions and primitive emotions. c. Its two major structures are the hippocampus and amygdala, essential for learning and memory. 1) The hippocampus makes prefrontal area aware of past experiences stored in association areas. 2) The amygdala causes experiences to have emotional overtones. 3) Inclusion of the frontal lobe in the limbic system allows reasoning to keep us from acting out strong feelings. d. Learning and Memory 1) Memory is the ability to hold thoughts in the mind and to recall past events. 2) Learning takes place when we retain and utilize past memories. 3) The prefrontal area in the frontal lobe is active in short-term memory (e.g., telephone numbers). 4) Long-term memory is a mix of semantic memory (numbers, words) and episodic memory (persons, events). 5) Skill memory is the ability to perform motor activities. 6) The hippocampus serves as a go-between to bring memories to mind. 7) The amygdala is responsible for fear conditioning and associates danger with sensory stimuli. 8) Long-term potentiation (LTP) is an enhanced response at synapses within the hippocampus. 9) LTP is essential to memory storage; excited postsynaptic cells may die due to a glutamate neurotransmitter. 10) Extinction of too many cells in the hippocampus is the underlying cause of Alzheimer disease. 25.4 Peripheral Nervous System 1. The peripheral nervous system lies outside the CNS. a. Cranial nerves connect to the brain. b. Spinal nerves lie on either side of the spinal cord. 2. Axons in nerves are called nerve fibers. 3. The cell bodies of neurons are found in the CNS or in ganglia. 4. Ganglia are collections of cell bodies in the PNS. 5. Humans have 12 pairs of cranial nerves attached to the brain. a. Sensory nerves only contain sensory nerve fibers. b. Motor nerves only contain motor nerve fibers. c. Mixed nerves contain both sensory and motor nerve fibers. d. Cranial nerves mostly connect to the head, neck, and facial regions. e. The vagus nerve also branches to the pharynx, larynx, and some internal organs. 6. Humans have 31 pairs of spinal nerves emerging from the spinal cord. a. The paired spinal nerves leave the spinal cord by two short branches, or roots. b. The dorsal root contains fibers of sensory neurons conducting nerve impulses to the spinal cord; the cell body of a sensory neuron is in the dorsal root ganglion. c. The ventral root contains the axons of motor neurons that conduct nerve impulses away from the spinal cord. d. All spinal nerves are mixed nerves that conduct impulses to and from the spinal cord. e. Spinal nerves are mixed nerves with sensory and motor fibers; each serves its own region. A. Somatic System 1. The somatic system includes the nerves that carry sensory information to the CNS and motor commands away from the CNS to skeletal muscles. 2. Any voluntary control of muscles involves the brain; reflexes, involuntary responses to 98 stimuli, involve the brain or just the spinal cord. 3. Outside stimuli can initiate reflex actions, some of which involve the brain. B. The Reflex Arc 1. Reflexes are automatic, involuntary responses. 2. A reflex arc involves the following pathway: a. Sensory receptors generate an impulse in a sensory neuron that moves along sensory axons toward the spinal cord. b. Sensory neurons enter the cord dorsally and pass signals to interneurons. c. Impulses travel along motor axons to an effector, which brings about a response to the stimulus. d. The immediate response is that muscles contract to withdraw from source of pain. 3. Reflex response occurs because the sensory neuron stimulates several interneurons. 4. Some impulses extend to the cerebrum, which makes a person conscious of the stimulus and the reaction. C. Autonomic System 1. The autonomic system is a part of the PNS and regulates cardiac and smooth muscle and glands. 2. There are two divisions: the sympathetic and parasympathetic systems. a. Both function automatically and usually in an involuntary manner. b. Both innervate all internal organs. c. Both utilize two neurons and one ganglion for each impulse. 1) The first neuron has a cell body within the CNS and a preganglionic fiber. 2) The second neuron has a cell body within the ganglion and a postganglionic fiber. d. Breathing rate and blood pressure are regulated by reflex actions to maintain homeostasis. 3. Sympathetic Division a. Most preganglionic fibers of the sympathetic system arise from the middle (thoracic-lumbar) portion of the spinal cord and almost immediately terminate in ganglia that lie near the cord (thoracic-lumbar portion). b. Therefore the preganglionic fiber is short, but the postganglionic fiber that contacts an organ is long. c. The sympathetic system is especially important during emergency situations (the “fight or flight” response). d. To defend or flee, muscles need a supply of glucose and oxygen; the sympathetic system accelerates heartbeat, and dilates bronchi. e. To divert energy from less necessary digestive functions, the sympathetic system inhibits digestion. f. The neurotransmitter released by the postganglionic axon is mainly norepinephrine, similar to epinephrine (adrenaline) used as a heart stimulant. 4. Parasympathetic Division a. The parasympathetic system consists of a few cranial nerves, including the vagus nerve, and fibers that arise from the bottom craniosacral portion of the spinal cord. b. In this case, the preganglionic fibers are long and the postganglionic fibers are short. c. This system is a “housekeeper system”; it promotes internal responses resulting in a relaxed state. d. The parasympathetic system causes the eye pupil to constrict, promotes digestion, and retards heartbeat. e. The neurotransmitter released is acetylcholine. Critical Thinking Question 1. What keeps a nerve impulse from flowing “backward” in a neuron and across a synapse? Question 2. Why do most people remember where they were when they heard the space shuttle Challenger had exploded or (for older students) when President Kennedy was shot? Question 3. How can a person be declared “brain dead” when he/she is still breathing? 99 Question 4. Why is it difficult to diagnose the cause of an inability to speak? 100 12.1, 13.1 - 13.6 Darwin and Evidence of Evolution CHAPTER 1 History of Evolutionary Thought 1. 2. In 1831, Charles Darwin, a 22-year-old naturalist, accepted a position aboard the ship HMS Beagle that began a voyage around the world; it provided Darwin with many observations. The pre-Darwinian world-view was different from the post-Darwinian. a. Pre-Darwinian world-view was determined by intractable theological beliefs. 1) The earth is young. 2) Each species was specially created and did not change over time. 3) Variations are imperfections varying from a perfectly-adapted creation. 4) Observations are to substantiate the prevailing worldview. b. Darwin, however, lived during a time of great change in scientific and social realms. c. Darwin’s ideas were part of a larger change in thought already underway among biologists; this concept would eventually be known as evolution. A. Mid-Eighteenth-Century Contributions 1. Carolus Linnaeus and Taxonomy a. Taxonomy is the science of classifying organisms; taxonomy had been a main concern of biology. b. Carolus Linnaeus (1707–1778) was a Swedish taxonomist. 1) Linnaeus developed a binomial system of nomenclature (two-part names for each species [e.g., Homo sapiens]). 2) He developed a system of classification for all known plants. 3) Like other taxonomists of his time, Linnaeus believed in the ideas of a) special creation—each species had an “ideal” structure and function; and b) fixity of species—each species had a place in the scala naturae, a sequential ladder of life. c. Linnaeus thought that classification should describe the fixed features of species and reveal God’s divine plan. d. His ideas reflected the ideas of Plato and Aristotle: the ideal form can be deduced, and organisms can be arranged in order of increasing complexity. e. His later work with hybridization suggested species might change with time. 2. Georges Louis Leclerc a. Georges Louis Leclerc, known by his title, Count Buffon (1707–1788), was a French naturalist. b. He wrote a 44-volume natural history of all known plants and animals. c. He also provided evidence of descent with modification. d. His writings speculated on influences of the environment, migration, 101 geographical isolation, and the struggle for existence. e. Buffon vacillated on whether he believed in evolutionary descent and he professed to believe in special creation and the fixity of species. 3. Erasmus Darwin a. Erasmus Darwin (1731–1802) was Charles Darwin’s grandfather. b. He was a physician and a naturalist whose writings on both botany and zoology contained many comments that suggested the possibility of common descent. c. He based his conclusions on 1) changes undergone by animals during development, 2) artificial selection by humans, and 3) the presence of vestigial organs (organs that are believed to have been functional in an ancestor but are reduced and nonfunctional in a descendant). d. Erasmus Darwin offered no mechanism by which evolutionary descent might occur. B. Late Eighteenth-/Early-Nineteenth Century Contributions 1. Cuvier and Catastrophism a. George Cuvier (1769–1832), a French vertebrate zoologist, was the first to use comparative anatomy to develop a system of classifying animals. b. He founded the science of paleontology—the study of fossils—and suggested that a single fossil bone was all he needed to deduce the entire anatomy of an animal. c. To explain the fossil record, Cuvier proposed that a whole series of catastrophes (extinctions) and re-populations from other regions had occurred. d. Cuvier was also a staunch advocate of special creation and fixity of species; this presented him with a problem when geological evidence of a particular region showed a succession of life forms in the earth’s strata. e. Catastrophism is the term applied to Cuvier’s explanation of fossil history: the belief that catastrophic extinctions occurred, after which repopulation of surviving species occurred, giving an appearance of change through time. 2. Lamarck’s Acquired Characteristics a. Lamarck (1744–1829) was the first to state that descent with modification occurs and that organisms become adapted to their environments. b. Lamarck, an invertebrate zoologist, held ideas at odds with Cuvier’s. c. Lamarck mistakenly saw “a desire for perfection” as inherent in all living things. d. Inheritance of acquired characteristics was Lamarck’s belief that organisms become adapted to their environment during their lifetime and pass these adaptations to their offspring. e. Experiments fail to uphold Lamarck’s inheritance of acquired characteristics; the molecular mechanism of inheritance shows phenotypic changes do not result in genetic changes that can be passed on to the next generation. 2 Darwin’s Theory of Evolution 102 A. Darwin’s Background 1. His nature was too sensitive to pursue medicine; he attended divinity school at Cambridge. 2. He attended biology and geology lectures and was tutored by the Reverend John Henslow. 3. Henslow arranged his five-year trip on the HMS Beagle; Darwin was an observant student of nature. B. Geology and Fossils 1. His study of geology and fossils caused him to concur with Lyell that the observed massive geological changes were caused by slow, continuous processes. a. Darwin took Lyell’s book on the voyage of the HMS Beagle. b. In his book Principles of Geology, Charles Lyell presented arguments to support a theory of geological change proposed by James Hutton. c. In contrast to catastrophists, Hutton proposed that the earth was subject to slow but continuous geological processes (e.g., erosion and uplifting) that occur at a uniform rate, a theory called uniformitarianism. d. The Argentina coast had raised beaches; he witnessed earthquakes raising the earth several feet. e. Marine shells occurred far inland and at great heights in the Andes. f. Fossils of huge sloths and armadillo-like animals suggested modern forms were descended from extinct forms with change over time; therefore species were not fixed. C. Biogeography 1. Biogeography is the study of the geographic distribution of life forms on earth. 2. Patagonian hares replaced rabbits in the South American grasslands. 3. The greater rhea found in the north was replaced by the lesser rhea in the south. 4. Comparison of the animals of South America and the Galápagos Islands caused Darwin to conclude that adaptation to the environment can cause diversification, including origin of new species. 5. The Galápagos Islands a. These volcanic islands off the South American coast had fewer types of organisms. b. Island species varied from the mainland species, and from island-to-island. c. Each island had a variation of tortoise; long and short necked tortoises correlated with different vegetation. d. Darwin’s Finches 1) Finches on the Galápagos Islands resembled a mainland finch but there were more types. 2) Galápagos finch species varied by nesting site, beak size, and eating habits. 3) One unusual finch used a twig or thorn to pry out insects, a job normally done by (missing) woodpeckers (Darwin never witnessed this finch behavior). 4) The variation in finches posed questions to Darwin: did they descend from one mainland ancestor or did islands allow isolated populations to evolve independently, and could present-day species have resulted from changes occurring in each isolated population? D. Natural Selection and Adaptation 1. Darwin decided that adaptations develop over time; he sought a mechanism by which adaptations might arise. 2. Natural selection was proposed by both Alfred Russel Wallace and Darwin as a driving mechanism of evolution caused by environmental selection of organisms most fit to 103 3. 4. reproduce, resulting in adaptation. Because the environment is always changing, there is no perfectly-adapted organism. There are three preconditions for natural selection. a. The members of a population have random but heritable variations. b. In a population, many more individuals are produced each generation than the environment can support. c. Some individuals have adaptive characteristics that enable them to survive and reproduce better. 5. There are two consequences of natural selection. a. An increasing proportion of individuals in succeeding generations will have the adaptive characteristics. b. The result of natural selection is a population adapted to its local environment. 6. Natural selection can only utilize variations that are randomly provided; therefore there is no directedness or anticipation of future needs. 7. Extinction occurs when previous adaptations are no longer suitable to a changed environment. E. Organisms Have Variations 1. In contrast to the previous worldview where imperfections were to be ignored, variations were essential in natural selection. 2. Darwin suspected, but did not have today’s evidence, that the occurrence of variation is completely random. 3. New variations are as likely to be harmful as helpful. 4. Variations that make adaptation possible are those that are passed on from generation to generation. 5. Darwin could not state the cause of variations because genetics was not yet established. F. Organisms Struggle to Exist 1. Darwin and Wallace both read an essay by Thomas Malthus, a socioeconomist. 2. Malthus proposed that human populations outgrow food supply and death and famine were inevitable. 3. Darwin applied this to all organisms; resources were not sufficient for all members to survive. 4. Therefore, there is a constant struggle for existence; only certain members survive and reproduce. G. Organisms Differ in Fitness 1. Organisms whose traits enable them to reproduce to a greater degree have a greater fitness. a. Fitness is a measure of an organism’s reproductive success. b. Black western diamondback rattlesnakes are more likely to survive on lava flows; lighter-colored rattlesnakes are more likely to survive on desert soil. 2. Darwin noted that humans carry out artificial selection. a. Early humans likely selected wolf variants; consequently, desirable traits increase in frequency in subsequent generations and produced the varieties of domestic dogs. b. Many crop plant varieties can be traced to a single ancestor. c. In nature, interactions with the environment determine which members reproduce more. d. Evolution by artificial or natural selection occurs when more fit organisms reproduce and leave more offspring than the less fit. H. Organisms Become Adapted 1. An adaptation is a trait that helps an organism be more suited to its environment. 2. Unrelated organisms living in the same environment often display similar characteristics. 3. Because of differential reproduction, adaptive traits increase in each succeeding generation. I. On the Origin of Species by Darwin 104 1. 2. 3. After the HMS Beagle returned to England in 1836, Darwin waited over 20 years to publish. He used the time to test his hypothesis that life forms arose by descent from a common ancestor and that natural selection is a mechanism by which species can change and new species arise. Darwin was forced to publish Origin of Species after reading a similar hypothesis by Alfred Russel Wallace. 3 The Evidence of Evolution A. Common Descent 1. The hypothesis of common descent is supported by many lines of evidence. 2. The more varied the evidence, the more certain it becomes. 3. Darwin synthesized much of the current data but biochemical research was yet to come. B. Fossils Evidence 1. The fossil record is the history of life recorded by remains from the past. 2. Fossils are at least 10,000 years old and include skeletons, shells, seeds, insects trapped in amber, and imprints of leaves. 3. The fossil record traces history of life and allows us to study history of particular organisms. 4. Fossil evidence supports the common descent hypothesis; fossils can be linked over time because they reveal a similarity in form, despite observed changes. 5. Transitional forms reveal links between groups. a. b. c. d. 6. Archeopteryx is an intermediate between reptiles and birds. Eustheopteron is an amphibious fish. Seymouria is a reptile-like amphibian. Therapsids were mammal-like reptiles. The fossil record allows us to trace the history of the modern-day horse Equus. a. Earliest fossils show an ancestral Hyracotherium the size of a dog, with cusped low-crowned molars, four toes on each front foot, three on each hind foot—all adaptations for forest living. b. When forests were replaced by grasslands, the intermediates were selected for durable grinding teeth, speed, etc. with an increase in size and decrease in toes. c. Living organisms resemble most recent fossils in the line of descent; underlying similarities allow us to trace a line of descent over time. C. Biogeographical Evidence 1. Biogeography studies the distribution of plants and animals worldwide. 2. Distribution of organisms is explained by related forms evolving in one locale and spreading to other accessible areas. a. Darwin observed South America had no rabbits; he concluded rabbits originated elsewhere. b. Biogeography explains the abundance of finch species on the Galápagos Islands lacking on the mainland. 3. Physical factors, such as the location of continents, determine where a population can spread. a. Cacti are restricted to North American deserts and euphorbia grow in African deserts. b. Marsupials arose when South America, Antarctica, and Australia were joined; Australia separated before placental mammals arose, so only marsupials diversified in Australia. D. Anatomical Evidence 1. Organisms have anatomical similarities when they are closely related because of common descent. a. Homologous structures in different organisms are inherited from a common ancestor. 105 b. Analogous structures are inherited from unique ancestors and have come to resemble each other because they serve a similar function. c. Vertebrate forelimbs contain the same sets of bones organized in similar ways, despite their dissimilar functions. 2. Vestigial structures are remains of a structure that was functional in some ancestors but is no longer functional in the organism in question. a. Most birds have well-developed wings; some bird species have reduced wings and do not fly. b. Humans have a tailbone but no tail. c. Presence of vestigial structures is explained by the common descent hypothesis; these are traces of an organism’s evolutionary history. 3. Embryological development reveals a unity of plan. a. During development, all vertebrates have a post-anal tail and paired pharyngeal pouches. 1) In fishes and amphibian larvae, the pouches become gills. 2) In humans, first pair of pouches becomes a cavity of middle ear and auditory tube; second pair becomes tonsils, while third and fourth pairs become thymus and parathyroid glands. 3) The above features are explained if fishes are ancestral to other vertebrate groups. E. Biochemical Evidence 1. Almost all living organisms use the same basic biochemical molecules, e.g., DNA, ATP, and many identical or nearly identical enzymes. 2. Organisms utilize the same DNA triplet code and the same 20 amino acids in their proteins. 3. Many organisms share the same introns and types of repeats, which is remarkable since there is no obvious functional reason why these components need to be so similar. 4. This is substantiated by the analysis of the degree of similarity in amino acids for cytochrome c among organisms. 5. These similarities can be explained by descent from a common ancestor. 6. Life’s vast diversity has come about by only a slight difference in the same genes. F. Because it is supported by so many lines of evidence, evolution is no longer considered a hypothesis. 1. Evolution is one of the great unifying theories of biology, similar in status to the germ theory of disease in medicine. 2. In science, theory is reserved for those conceptual schemes that are supported by a large number of observations or a large amount of experimental evidence and have not been found lacking. Critical Thinking Question 1. If there is no variation in a population, or if the only variation is acquired and not inherited, or if all of the progeny survive and equally reproduce, will evolution occur? Question 2. Why are an insect wing and a bird wing not considered evidence of relatedness? Question 3. If you have a cut on your finger that leaves a permanent scar, this readily shows in fingerprinting and does distinguish you from the rest of the human population. Will such a variation become part of the evolutionary fate of your lineage through natural selection?Question 4. If a wolf loses a leg in a trap and this cripples its ability to hunt, it 106 has far less chance of leaving offspring. Will that variation become part of the evolutionary fate of its lineage through natural selection? 107 Chapter 12, 13 Process of Evolution 1 Microevolution 1. 2. 3. 4. 5. 6. 7. It was not until the 1930s that population geneticists were able to apply the principles of genetics to populations and thus to recognize when evolution had occurred. A population is all of the members of a single species occupying a certain area at the same time. Evolution that occurs within a population is called microevolution. Population genetics studies the variation in alleles in a gene pool. The gene pool is the total of all the alleles in a population; it is described in terms of gene frequencies. Neither dominance nor sexual reproduction changes allele frequencies. The Hardy-Weinberg principle a. This principle states an equilibrium of allele frequencies in a gene pool (using a formula p2 + 2pq + q2) remains in effect in each succeeding generation of a sexually reproducing population if five conditions are met. 1) No mutation: no allelic changes occur, or changes in one direction are balanced by changes in the other direction. 2) No gene flow: migration of alleles into or out of the population does not occur. 3) Random mating: individuals pair by chance and not according to their genotypes or phenotypes. 4) No genetic drift: the population is large so changes in allele frequencies due to chance are insignificant. 5) No selection: no selective force favors one genotype over another. b. In real life, conditions of the Hardy-Weinberg law are rarely if ever met, and allele frequencies in the gene pool of a population do change from one generation to the next, resulting in evolution. c. Any change of allele frequencies in a gene pool of a population signifies that evolution has occurred. d. The Hardy-Weinberg law tells us what factors cause evolution—those that violate the conditions listed. e. A Hardy-Weinberg equilibrium provides a baseline by which to judge whether evolution has occurred. f. Hardy-Weinberg equilibrium is a constancy of gene pool frequencies that remains across generations. 8. Industrial Melanism a. The case of the peppered moths provides a case study in a shift in phenotype frequencies under selection. b. Before trees became coated with soot from air pollution, the percentage of dark-colored moths was 10%. c. With birds acting as a selective agent, the light colored moths were reduced while dark-colored moths were better adapted to survive on the darkened trees. 108 d. The last generation observed has 80% dark-colored moths. A. Causes of Microevolution 1. Genetic Mutations a. Many traits in organisms are polymorphic, i.e., two or more distinct phenotypes are present in the population due to mutated genes. b. Analysis of Drosophila enzymes indicates they have multiple alleles at least at 30% of their gene loci. c. In humans, freckles are an example of polymorphism, as are the ABO blood types. d. e. Mutations may not immediately affect the phenotype. Mutations can be beneficial, neutral, or harmful; a seemingly harmful mutation that requires Daphnia to live at higher temperatures becomes advantageous when the environment changes. Specific recombinations of alleles may be more adaptive than other combinations. f. B. Gene Flow 1. Gene flow (gene migration) is the movement of alleles among populations by migration of breeding individuals. 2. Gene flow can increase variation within a population by introducing novel alleles produced by mutation in another population. 3. Continued gene flow decreases diversity among populations, causing gene pools to become similar. 4. Gene flow among populations can prevent speciation from occurring. C. Nonrandom Mating 1. Random mating involves individuals pairing by chance, not according to genotype or phenotype. 2. Nonrandom mating involves inbreeding and assortative mating. 3. Inbreeding is mating between relatives to a greater extent than by chance. a. Inbreeding does not change the allele frequencies. b. However, inbreeding decreases the proportion of heterozygotes. c. Inbreeding increases the proportions of both homozygotes at all gene loci. d. In human populations, inbreeding increases the frequency of recessive abnormalities. 4. Assortative mating occurs when individuals mate with those that have the same phenotype. a. Assortative mating divides a population into two phenotypic classes with reduced gene exchange. b. Homozygotes for gene loci that control a trait increase, and heterozygotes for these loci decrease. 5. Sexual selection occurs when males compete for the right to reproduce and the female selects males of a particular phenotype. D. Genetic Drift 1. Genetic drift refers to changes in allele frequencies of a gene pool due to chance. 2. Genetic drift occurs in both large and small populations; large populations suffer less sampling error. 3. Genetic drift causes isolated gene pools to become dissimilar; some alleles are lost and others are fixed or are the only allele in the population. 4. Genetic drift occurs when founders start a new population, or after a genetic bottleneck with interbreeding. a. The bottleneck effect prevents most genotypes from participating in production of the next generation. 1) The bottleneck effect is caused by a severe reduction in population size due to a natural disaster, predation, or habitat reduction. 2) The bottleneck effect causes a severe reduction in the total genetic diversity of the original gene pool. 109 3) The cheetah bottleneck causes relative infertility because alleles were lost due to intense inbreeding when populations were reduced in earlier times. b. The founder effect is an example of genetic drift where rare alleles or combinations occur in higher frequency in a population isolated from the general population. 1) This is due to founding individuals containing a fraction of total genetic diversity of the original population. 2) Which particular alleles are carried by the founders is dictated by chance alone. 3) As an example, dwarfism is much higher in a Pennsylvania Amish community due to a few German founders. 2 Natural Selection Natural selection is the process that results in adaptation of a population to the environment. 1. Natural selection requires a. variation (i.e., the members of a population differ from one another), b. inheritance (i.e., many of the differences between individuals in a population are heritable genetic differences), c. differential adaptedness (i.e., some differences affect how well an organism is adapted to its environment), and d. differential reproduction (i.e., better adapted individuals are more likely to reproduce). 2. Fitness is the extent to which an individual contributes fertile offspring to the next generation. 3. Relative fitness compares the fitness of one phenotype to another. A. Types of Selection. 1. Directional selection occurs when an extreme phenotype is favored; the distribution curve shifts that direction. a. b. c. d. 2. A shift to dark-colored peppered moths from light-colored correlated with increasing pollution. Drug-resistant strains of bacteria are a serious health threat and represent this type of selection. Increases in insecticide-resistant mosquitoes and resistance of the malaria protozoan Plasmodium to medications are also examples of directional selection. The gradual increase in the size of the modern horse, Equus, correlates with a change in the environment from forest-like conditions to grassland conditions. Stabilizing selection occurs when extreme phenotypes are eliminated and the intermediate phenotype is favored. a. The average number of eggs laid by Swiss starlings is four or five. b. If the female lays more or less than this number, fewer survive. c. Genes determining the physiology of yolk production and behavior are involved in clutch size. 3. Disruptive selection occurs when extreme phenotypes are favored and can lead to more than one distinct form. a. British snails (Cepaea nemoralis) vary because a wide range causes natural selection to vary. 110 b. In forest areas, thrushes feed on snails with light bands. c. In low-vegetation areas, thrushes feed on snails with dark shells that lack light bands. B. Maintenance of Variations 1. Populations always show some genotypic variation; populations that lack variation may not be able to adapt to new conditions. 2. How is variation maintained in the face of constant selection pressure? 3. The following forces promote genetic variation. a. Mutation creates new alleles and genetic recombination still combines these alleles. b. Gene flow among small populations introduces new alleles. c. Natural selection, such as disruptive selection, itself sometimes promotes variation. 4. Diploidy and the Heterozygote a. Only alleles that cause phenotypic differences are subject to natural selection. b. In diploid organisms, a heterozygote shelters rare recessive alleles that would otherwise be selected out. c. Even when selection reduces the recessive allele frequency from 0.9 to 0.1, the frequency in the heterozygote remains the same and remains a resource for natural selection in a new environment. 5. Sickle-Cell Disease a. In sickle-cell disease, heterozygotes are more fit in malaria areas because the sickle-cell trait does not express unless the oxygen content of the environment is low; but the malaria agent causes red blood cells to die when it infects them (loss of potassium). b. Some homozygous dominants are maintained in the population but they die at an early age from sickle-cell disease. c. Some homozygotes are maintained in the population for normal red blood cells, but they are vulnerable to malaria. 3. Macroevolution Macroevolution refers to any evolutionary change at or above the species level. Speciation is the splitting of one species into two or more species or the transformation of one species into a new species over time; speciation is the final result of changes in gene pool allele and genotypic frequencies. A. What is a Species? 1. Linnaeus separated species based on morphology, i.e., their traits differed; Darwin saw that similar species are related by common descent. 2. Ernst Mayr (1942) developed the biological species concept: a species is a group of actually or potentially interbreeding populations that are reproductively isolated from other such groups. 3. The biological definition of a species says that the members of one species interbreed and have a shared gene pool, and each species is reproductively isolated from every other species. 4. Gene flow occurs between populations of one species but not between populations of different species. 5. Biochemical genetics uses DNA hybridization techniques to determine relatedness of organisms; the phylogenetic species concept uses DNA/DNA comparisons. B. Reproductive Isolating Mechanisms 1. For two species to be separate, gene flow must not occur between them. 2. A reproductive isolating mechanism is any structural, functional, or behavioral characteristic that prevents successful reproduction from occurring. 3. Prezygotic (“before formation of a zygote”) isolating mechanisms are anatomical or behavioral differences between the members of two species that prevent mating or make it 111 unlikely fertilization will take place if mating occurs. a. Habitat isolation occurs when two species occupy different habitats, even within the same geographic range, so that they are less likely to meet and to attempt to reproduce. b. Temporal isolation occurs when two species live in the same location, but each reproduces at a different time of year, and so they do not attempt to mate. c. Behavioral isolation results from differences in mating behavior between two species. d. Mechanical isolation is the result of differences between two species in reproductive structures or other body parts, so that mating is prevented. e. Gamete isolation includes incompatibility of gametes of two different species so they cannot fuse to form a zygote; an egg may have receptors only for the sperm of its own species or a plant stigma prevents completion of pollination. 4. Postzygotic (“after formation of a zygote”) isolating mechanisms prevent development of a hybrid after mating has taken place. a. Zygote mortality is when hybrids (offspring of parents of two different species) do not live to reproduce. b. Hybrid sterility occurs when the hybrid offspring are sterile (e.g., mules). c. In F2 fitness, the offspring are fertile but the F2 generation is sterile. C. Modes of Speciation 1. Allopatric speciation occurs when new species result from populations being separated by a geographical barrier that prevents their members from reproducing with each other. a. First proposed by Ernst Mayr of Harvard University. b. While geographically isolated, variations accumulate until the populations are reproductively isolated. c. First postzygotic isolation occurs, then prezygotic reproductive isolation occurs. 2. Sympatric speciation would occur when members of a single population develop a genetic difference (e.g., chromosome number) that prevents them from reproducing with the parent type. a. The main example of sympatric speciation is in plants. b. Failure to reduce chromosome number produces polyploid plants that reproduce successfully only with polyploids. c. Backcrosses with diploids are sterile. D. Adaptive Radiation 1. Adaptive radiation is a rapid development from a single ancestral species of many new species. 2. The case of Darwin’s finches illustrates the adaptive radiation of 13 species from one founder mainland finch. 3. On the Hawaiian Islands, a wide variety of honeycreepers descended from one goldfinchlike ancestor; Hawaii is also the home of the silversword plants that radiated from ancestral tarweeds. Critical Thinking Question 1. Most organisms have a surprising amount of genetic diversity, or potential for variation, within their gene pool. Only some of them are best adapted for the exact 112 environmental conditions in your local area this year. Why does the population possess all of this genetic variation? Question 2. Consider a time period of continual variation in environmental conditions that is then followed by a time period of relative stability and lack of variation. Compare this to the opposite, a time period of stability followed by a time period of continual environmental fluctuation. In which case would you expect to see more species go extinct? Question 3. Consider the proposal that left-handedness is inherited as a recessive gene (rr) and right-handedness is dominant (RR, Rr). If 16% of this class is left-handed, what proportion of the right-handed students are likely to be carriers of a left-handedness gene and what is the frequency of the hypothetical left-handed gene? 113 CHAPTER 14.2, 14.5 VIRUSES, BACTERIA AND ARCHAEA 1 Viruses, Viroids, and Prions Viruses 1. are associated with a number of plant, animal, and human diseases; 2. can only reproduce by using the metabolic machinery of the host cell; 3. are noncellular; 4. may have a DNA or RNA genome. 5. In 1884, Pasteur suspected something smaller than bacteria caused rabies; he chose a Latin term for “poison.” 6. In 1892, Russian biologist Dimitri Ivanowsky, working with the tobacco mosaic virus, confirmed Pasteur’s hypothesis that an infectious agent smaller than a bacterium existed. 7. With the invention of the electron microscope, these infectious agents could be seen for the first time. A. Viral Structure 1. 2. 3. 4. A virus is similar in size to a large protein, generally smaller than 200 nm in diameter. Many viruses can be purified and crystallized, and the crystals stored for long periods of time. Viral crystals become infectious when the viral particles they contain invade host cells. All viruses have at least two parts: a. An outer capsid is composed of protein subunits. b. An inner core contains either DNA (deoxyribonucleic acid) or RNA (ribonucleic acid), but not both. 1) The viral genome at most has several hundred genes; a human cell, in comparison, contains thousands of genes. 2) The viral envelope is usually partly host plasma membrane with viral glycoprotein spikes. 3) Viral particles have proteins, especially enzymes (e.g., polymerases), to produce viral DNA or RNA. 4) Not all viruses have an envelope; such viruses are called naked viruses. 5. The classification of viruses is based on a. their type of nucleic acid, including whether they are single-stranded or double-stranded; b. their size and shape; and c. the presence or absence of an outer envelope. B. Parasitic Nature 1. Viruses are obligate intracellular parasites that cannot multiply outside a living cell. a. Animal viruses in laboratories are raised in live chick embryos or in cell tissue culture. b. Viruses infect all sorts of cells, from bacteria to human cells, but they are host specific. 1) The tobacco mosaic virus only infects certain plants. 2) The rabies virus infects only mammals. 3) The AIDS virus, HIV, infects only certain human blood cells. 4) The Hepatitis virus invades only liver tissues. 5) The Polio virus only reproduces in spinal nerve cells. 2. Virus Evolution a. Some believe that viruses originated from the very cells that they infect. b. For example, viral nucleic acids originated from the host cell genome. c. Therefore, viruses evolved after cells came into existence; new viruses are probably evolving now. 114 d. 3. Others suggest that viruses arose before the three domains. Viruses often mutate; therefore, it is correct to say that they evolve. a. Those that mutate are troublesome; a vaccine effective today may not be effective tomorrow. b. Influenza (flu) viruses mutate regularly. C. Viral Reproduction 1. Viruses gain entry into and are specific to a particular host cell because portions of the capsid (or spikes of the envelope) adhere to specific receptor sites on the host cell surface. 2. Viral nucleic acid then enters a cell, where viral genome codes for production of protein units in the capsid. 3. A virus may have genes for a few special enzymes needed for the virus to reproduce and exit from a host cell. 4. A virus relies on host cell enzymes, ribosomes, transfer RNA (tRNA), and ATP for its own replication. D. Reproduction of Bacteriophages 1. Bacteriophages (phages) are viruses that parasitize bacteria. 2. The lytic cycle is a bacteriophage’s “life” cycle consisting of five stages: a. During attachment, portions of the capsid bind with receptors on the bacterial cell wall. b. During penetration, a viral enzyme digests part of cell wall; the viral DNA is injected into a bacterial cell. c. Biosynthesis involves synthesis of viral components and begins after the virus brings about inactivation of host genes not necessary to viral replication. d. During maturation, viral DNA and capsids are assembled to produce several hundred viral particles and lysozyme, coded by the virus, is produced. e. When lysozyme disrupts the cell wall, release of the viral particles occurs and the bacterial cell dies. 3. With the lysogenic cycle, the virus incorporates its DNA into the bacterium but only later is phage produced. a. Following attachment and penetration, viral DNA becomes integrated into bacterial DNA with no destruction of the host DNA. b. At this point, the phage is latent and the viral DNA is called a prophage. c. The prophage is replicated along with host DNA; all subsequent cells (lysogenic cells) carry a copy. d. Certain environmental factors (e.g., ultraviolet radiation) induce prophage to enter the biosynthesis stage of the lytic cycle, followed by maturation and release. E. Reproduction of Animal Viruses 1. Animal viruses replicate similarly to bacteriophages, but there are modifications. a. If the virus has an envelope, glycoprotein spikes allow it to adhere to plasma membrane receptors. b. The virus genome covered by the capsid penetrates the host cell. c. Once inside, the virus is uncoated as the envelope and capsid are removed. d. Free of its covering, the viral genome (DNA or RNA) proceeds with biosynthesis. e. Newly assembled viral particles are released by budding. f. Components of viral envelopes (i.e., lipids, proteins, and carbohydrates) are obtained from the plasma or nuclear membrane of the host cell as the viruses leave. 2. Retroviruses are RNA animal viruses that have a DNA stage. a. Retroviruses contain the enzyme reverse transcriptase that uses RNA as a template to produce cDNA; cDNA is is a copy of the viral genome. b. Viral cDNA is integrated into host DNA and is replicated as host DNA replicates. c. Viral DNA is transcribed; new viruses are produced by biosynthesis and maturation; release is by budding. F. Viral Infections of Special Concern 1. Viruses cause infectious diseases in plants and animals, including humans. 2. Some animal viruses are specific to human cells: papillomavirus, herpes virus, hepatitis virus, 115 3. 4. 5. 6. 7. 8. and adenoviruses, which can cause specific cancers. Retroviruses include the AIDS viruses (e.g., HIV) and also cause certain forms of cancer. Emerging Viruses HIV is an example of an emerging virus: the causative agent of a disease that has only recently arisen and infected people. In some cases of emerging diseases, the virus is simply transported from one location to another; e.g., West Nile virus and severe acute respiratory syndrome (SARS). The high mutation rate of viruses also cause infectious viruses to emerge; e.g., AIDS and Ebola fever. A change in the mode of transmission is yet another way infectious viruses could emerge. G. Viroids and Prions 1. Viroids are naked strands of RNA, a dozen of which cause crop diseases. 2. Like viruses, viroids direct the cell to produce more viroids. 3. Prions (proteinaceous infectious particles) are newly discovered disease agents that differ from viruses and bacteria. a. Prions are rogue proteins with a wrongly-shaped tertiary structure that cause other proteins to distort. b. Creutzfeldt-Jakob disease in humans and scrapie and mad cow disease (BSE) in cattle are due to prions. 2 The Prokaryotes Prokaryotes include the bacteria and archaea. 1. Bacteria were discovered in the seventeenth century when Antonie van Leeuwenhoek examined scrapings from his teeth. 2. The “little animals” Leeuwenhoek observed were thought by him and others to arise spontaneously from inanimate matter. 3. Around 1850, Pasteur devised an experiment showing that the bacteria present in air contaminated the media. 4. A single spoonful of soil contains 1010 prokaryotes; these are the most numerous life forms. A. Structure of Prokaryotes 1. Prokaryotes range in size from 1–10 µm in length and from 0.7–1.5 µm in width. 2. “Prokaryote” means “before a nucleus”—their cells lack a eukaryotic nucleus. 3. Prokaryotic fossils date back as far as 3.5—3.8 billion years ago. 4. Fossils indicate prokaryotes were alone on earth for 2 billion years; they evolved very diverse metabolic capabilities. 5. Prokaryotes adapted to most environments because they differ in the many ways they acquire and utilize energy. 6. Outside the plasma membrane of most cells is a rigid cell wall that keeps the cell from bursting or collapsing due to osmotic changes by peptidoglycan, a complex molecule containing a unique amino disaccharide and peptide fragments. a. The cell wall may be surrounded by an organized capsule called a glycocalyx and/or by a loose gelatinous sheath called a slime layer. b. In parasitic forms, these outer coverings protect the cell from host defenses. 7. Some prokaryotes move by means of flagella. a. The flagellum has a filament composed of three strands of the protein flagellin wound in a helix and inserted into a hook that is anchored by a basal body. b. The flagellum is capable of 360o rotation which causes the cell to spin and move forward. 8. Many prokaryotes adhere to surfaces by means of fimbriae. a. Fimbriae are short hairlike filaments extending from the surface. b. The fimbriae of Neisseria gonorrhoeae allow it to attach to host cells and cause gonorrhea. 9. Prokaryotic cells lack the membranous organelles of eukaryotic cells. 10. Various metabolic pathways are located on the plasma membrane. 11. A nucleoid is a dense area in prokaryotes where the chromosome is located; it is a single 116 circular strand of DNA. 12. Plasmids are accessory rings of DNA found in some prokaryotes; they can be extracted and used as vectors to carry foreign DNA into bacteria during genetic engineering procedures. 13. Protein synthesis in prokaryotic cells is carried out by thousands of ribosomes, which are smaller than eukaryotic ribosomes. B. Reproduction in Prokaryotes 1. Binary fission is the splitting of a parent cell into two daughter cells; it is asexual reproduction in prokaryotes. a. A single circular chromosome replicates; the two copies separate as the cell enlarges. b. Newly formed plasma membrane and the cell wall separate the cell into two cells. c. Mitosis, which involves formation of a spindle apparatus, does not occur in prokaryotes. d. Because prokaryotes have a short generation time, mutations are generated and distributed through a population more rapidly. e. Prokaryotes are haploid; mutations are therefore immediately subjected to natural selection. 2. In bacteria, genetic recombination can occur in three ways. a. Conjugation occurs when a bacterium passes DNA to a second bacterium through a tube (sex pilus) that temporarily joins two cells; this occurs only between bacteria in the same or closely related species. b. Transformation involves bacteria taking up free pieces of DNA secreted by live bacteria or released by dead bacteria. c. In transduction, bacteriophages transfer portions of bacterial DNA from one cell to another. d. Plasmids can carry genes for resistance to antibiotics and transfer them between bacteria by any of these processes. 3. Some bacteria form resistant endospores in response to unfavorable environmental conditions. a. Some cytoplasm and the chromosome dehydrate and are encased by three heavy, protective spore coats. b. The rest of the bacterial cell deteriorates and the endospore is released. c. Endospores survive in the harshest of environments: desert heat and dehydration, boiling temperatures, polar ice, and extreme ultraviolet radiation. d. Endospores also survive very long periods of time; anthrax spores 1,300 years old can cause disease. e. When environmental conditions are again suitable, the endospore absorbs water and grows out of its spore coat. f. In a few hours, newly emerged cells become typical bacteria capable of reproducing by binary fission. g. Endospore formation is not reproduction--it is a means of survival and dispersal to new locations. C. Prokaryotic Nutrition 1. Bacteria differ in their need for, and tolerance of, oxygen (O 2). a. Obligate anaerobes are unable to grow in the presence of O 2; this includes anaerobic bacteria that cause botulism, gas gangrene, and tetanus. b. Facultative anaerobes are able to grow in either the presence or absence of gaseous O 2. c. Aerobic organisms (including animals and most prokaryotes) require a constant supply of O2 to carry out cellular respiration. 2. Autotrophic Prokaryotes a. Photoautotrophs are photosynthetic and use light energy to assemble the organic molecules they require. 1) Primitive photosynthesizing bacteria (e.g., green sulfur bacteria and purple sulfur bacteria) use only photosystem I that contains bacteriochlorophyll; they do not give off O2 because hydrogen sulfide (H2S) is used as an electron and H+ donor instead of H2O. 2) Advanced photosynthesizing bacteria (e.g., cyanobacteria) use both photosystem I and II that contain the same types of chlorophylls found in plants; they do give off 117 O2 because H2O is used as an electron and H+ donor. b. Chemoautotrophs make organic molecules by using energy derived from the oxidation of inorganic compounds in the environment. 1) Deep ocean hydrothermal vents provide H2S to form chemosynthetic bacteria. 2) The methanogens are chemosynthetic bacteria that produce methane (CH 4) from hydrogen gas and CO2; ATP synthesis and CO2 reduction are linked to this reaction and methanogens can decompose animal wastes to produce electricity as an ecological friendly energy source. 3) Nitrifying bacteria oxidize ammonia (NH3) to nitrites (NO2) and nitrites to nitrates (NO3). 3. Heterotrophic Prokaryotes a. Most free-living bacteria are chemoheterotrophs that take in pre-formed organic nutrients. b. As aerobic saprotrophs, there is probably no natural organic molecule that cannot be broken down by some prokaryotic species. c. Detritivores (saprophytic bacteria) are critical in recycling materials in the ecosystem; they decompose dead organic matter and make it available to photosynthesizers. d. Prokaryotes produce chemicals including ethyl alcohol, acetic acid, butyl alcohol, and acetones. e. Prokaryotic action produces butter, cheese, sauerkraut, rubber, cotton, silk, coffee and cocoa. f. Antibiotics are produced by some bacteria. 4. Some chemoheterotrophs are symbiotic, forming relationships with members of other species; forms of symbiosis include mutualistic, commensalistic, and parasitic relationships. a. Mutualistic nitrogen-fixing Rhizobium bacteria live in nodules on roots of soybean, clover, and alfalfa where they reduce N2 to ammonia for their host; bacteria use some of a plant’s photosynthetically produced organic molecules. b. Mutualistic bacteria that live in the intestines of humans benefit from undigested material and release vitamins K and B12, which we use to produce blood components. c. In the stomachs of cows and goats, mutualistic prokaryotes digest cellulose. d. Commensalistic bacteria live in or on organisms of other species and cause them no harm. e. Parasitic bacteria are responsible for a wide variety of infectious plant, animal and human diseases. 5. Bacterial Diseases in Humans a. Microbes that cause disease are called pathogens. b. Pathogens may be able to produce a toxin, and or adhere to surfaces and sometimes invade organs or cells. 1) Toxins are small organic molecules, or small pieces of protein or parts of the bacterial cell wall, that are released when bacteria die. 2) In almost all cases, the growth of the bacteria does not cause disease but instead the toxins they release cause the disease. Example: Clostridium tetani, the causative agent of tetanus. c. Adhesion factors allow a pathogen to bind to certain cells, which determines which tissue in the body will be the host. Example: Shigella dysentariae releases a toxin and also sticks to the intestinal wall, making it a life-threatening form of dysentary. d. Antibacterial compounds either inhibit cell wall synthesis or protein biosynthesis; increasingly, many pathogenic bacteria are becoming resistant to bacteria. 3 The Bacteria 1. 2. The Gram stain procedure (developed in the late 1880s by Hans Christian Gram) differentiates bacteria. a. Gram-positive bacteria stain purple, whereas Gram-negative bacteria stain pink. b. This difference is dependent on the thick or thin (respectively) peptidoglycan cell wall. Bacteria and archaea have three basic shapes. a. A spirillum is spiral-shaped. 118 b. c. d. A bacillus is an elongated or rod-shaped bacteria. Coccus bacteria are spherical. Cocci and bacilli tend to form clusters and chains of a length typical of the particular species. 3. Other criteria for classification of bacteria are the presence of endospores, metabolism, growth and nutritional characteristics, and other physiological characteristics. 4. Recent work by Carl Woese has revised bacterial taxonomy based on similarity of 16S rRNA; twelve groups are now recognized based on bacterial 16S ribosomal RNA sequences. A. Cyanobacteria 1. Cyanobacteria are Gram-negative bacteria with a number of unusual traits. 2. They photosynthesize in the same manner as plants, and thus are responsible for introducing O2 into the primitive atmosphere. 3. They were formerly mistaken for eukaryotes and classified with algae. 4. Cyanobacteria have pigments that mask chlorophyll; they are not only blue-green but also red, yellow, brown, or black. 5. They are relatively large (1–50 µm in width). 6. They can be unicellular, colonial, or filamentous. 7. Some move by gliding or oscillating. 8. Some possess heterocysts, thick-walled cells without a nucleoid, where nitrogen fixation occurs. 9. Cyanobacteria are common in fresh water, soil, on moist surfaces, and in harsh habitats (e.g., hot springs). 10. Some species are symbiotic with other organisms (e.g., liverworts, ferns, and corals). 11. Lichens are a symbiotic relationship where the cyanobacteria provide organic nutrients to the fungus and the fungus protects and supplies inorganic nutrients. 12. Cyanobacteria were probably the first colonizers of land during evolution. 13. Cyanobacteria “bloom” when nitrates and phosphates are released as wastes into water; when they die off, decomposing bacteria use up the oxygen and cause fish kills. 4 The Archaea A. Relationship to Domain Bacteria and Domain Eukarya 1. Archaea are prokaryotes with molecular characteristics that distinguish them from bacteria and eukaryotes; their rRNA base sequence is different from that in bacteria. 2. Because archaea and some bacteria are both found in extreme environments (hot springs, thermal vents, salt basins), they may have diverged from a common ancestor. 3. Later, the eukarya split from the archaea; archaea and eukarya share some ribosomal proteins not found in bacteria; initiate transcription in the same manner, and have similar types of tRNAs. B. Structure and Function 1. 2. 3. 4. 5. Archaea have unusual lipids in their plasma membranes that allow them to function at high temperatures: glycerol linked to hydrocarbons rather than fatty acids. Cell walls of archaea do not contain the peptidoglycan found in bacterial cell walls. Only some methanogens have the ability to form methane. Most are chemoautotrophs; none are photosynthetic; this suggests chemoautotrophy evolved first. Some are mutualistic or commensalistic but none are parasitic—none are known to cause disease. C. Types of Archaea 1. 2. Methanogens live under anaerobic environments (e.g., marshes) where they produce methane. a. Methane is produced from hydrogen gas and carbon dioxide and is coupled to formation of ATP. b. Methane released to the atmosphere contributes to the greenhouse effect. c. About 65% of the methane found in our atmosphere is produced by methanogenic archaea. Halophiles require high salt concentrations (e.g., Great Salt Lake). a. Their proteins have unique chloride pumps that use halorhodopsin to synthesize ATP in 119 3. the presence of light. b. They usually require 12–15% salt concentrations; the ocean is only 3.5% salt. Thermoacidophiles live under hot, acidic environments (e.g., geysers). a. They survive best at temperatures above 80oC; some survive above boiling temperatures. b. Metabolism of sulfides forms acidic sulfates; these bacteria grow best at pH of 1 to 2. Critical Thinking Question 1. Why do we refer to viruses as “activated” or “inactivated” rather than “living” or “dead”? Question 2. Why is a chemical agent, such as sulfa or phenol, not considered an “antibiotic”? Question 3. Why are bacterial 16S ribosomal RNA sequences used rather than the circular chromosome loop in research that attempts to work out the phylogenetic relationships of prokaryotes? 120 121