Inorganic Qualitative Tests: Anion & Alkali Identification
advertisement
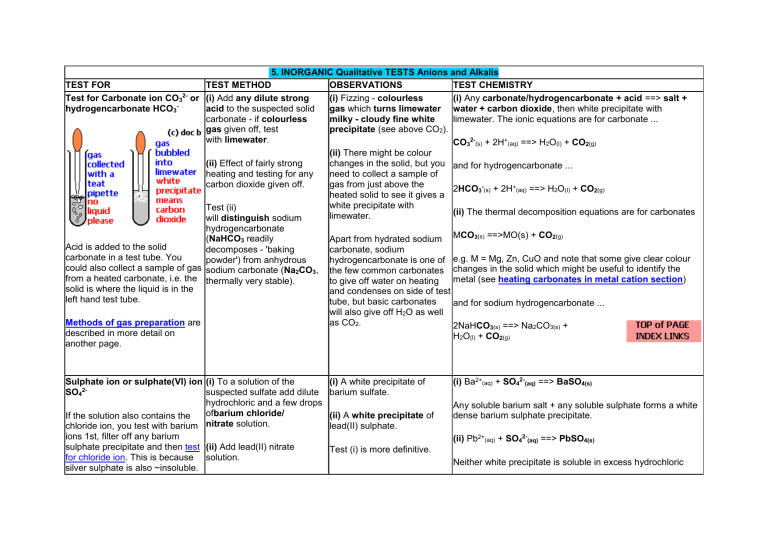
TEST FOR Test for Carbonate ion CO32- or hydrogencarbonate HCO3- Acid is added to the solid carbonate in a test tube. You could also collect a sample of gas from a heated carbonate, i.e. the solid is where the liquid is in the left hand test tube. Methods of gas preparation are described in more detail on another page. 5. INORGANIC Qualitative TESTS Anions and Alkalis TEST METHOD OBSERVATIONS TEST CHEMISTRY (i) Add any dilute strong (i) Fizzing - colourless (i) Any carbonate/hydrogencarbonate + acid ==> salt + acid to the suspected solid gas which turns limewater water + carbon dioxide, then white precipitate with carbonate - if colourless milky - cloudy fine white limewater. The ionic equations are for carbonate ... gas given off, test precipitate (see above CO2). with limewater. CO32-(s) + 2H+(aq) ==> H2O(l) + CO2(g) (ii) There might be colour (ii) Effect of fairly strong changes in the solid, but you and for hydrogencarbonate ... heating and testing for any need to collect a sample of carbon dioxide given off. gas from just above the 2HCO3-(s) + 2H+(aq) ==> H2O(l) + CO2(g) heated solid to see it gives a white precipitate with Test (ii) (ii) The thermal decomposition equations are for carbonates limewater. will distinguish sodium hydrogencarbonate (NaHCO3 readily Apart from hydrated sodium MCO3(s) ==>MO(s) + CO2(g) decomposes - 'baking carbonate, sodium powder') from anhydrous hydrogencarbonate is one of e.g. M = Mg, Zn, CuO and note that some give clear colour sodium carbonate (Na2CO3, the few common carbonates changes in the solid which might be useful to identify the metal (see heating carbonates in metal cation section) thermally very stable). to give off water on heating and condenses on side of test tube, but basic carbonates and for sodium hydrogencarbonate ... will also give off H2O as well as CO2. 2NaHCO3(s) ==> Na2CO3(s) + H2O(l) + CO2(g) Sulphate ion or sulphate(VI) ion (i) To a solution of the SO42suspected sulfate add dilute hydrochloric and a few drops ofbarium chloride/ If the solution also contains the chloride ion, you test with barium nitrate solution. ions 1st, filter off any barium sulphate precipitate and then test (ii) Add lead(II) nitrate for chloride ion. This is because solution. silver sulphate is also ~insoluble. (i) A white precipitate of barium sulfate. (ii) A white precipitate of lead(II) sulphate. (i) Ba2+(aq) + SO42-(aq) ==> BaSO4(s) Any soluble barium salt + any soluble sulphate forms a white dense barium sulphate precipitate. (ii) Pb2+(aq) + SO42-(aq) ==> PbSO4(s) Test (i) is more definitive. Neither white precipitate is soluble in excess hydrochloric acid. Sulphite ion or sulphate(IV) ion (i) Add dilute hydrochloric SO32acid to the suspected sulfite. Test (iii) is easily unreliable, the sulphite ion is oxidised by air (dissolved oxygen) to give the sulphate ion, so you will lucky to obtain a clear solution after adding excess acid. Sulphide ion S2- (i) Acrid choking sulfur dioxide gas formed. (ii) Test any gas evolved with (ii) The dichromate fresh potassium dichromate(VI) paper. paper turns from orangeto green. (iii) Add barium chloride or (iii) A white ppt. of barium barium nitrate solution. sulphite which dissolves in excess hydrochloric acid to give a clear colourless solution. (i) If soluble, add a few drops (i) Black precipitate of lead lead(II) ethanoate solution. sulphide. In test (ii) dangerous hydrogen sulphide is formed. (ii) If solid, add dil. HCl(aq) (ii) Rotten egg smell of acid, test smelly gas with hydrogen sulphide and the damp lead(II) ethanoate H2S gas turns lead(II) paper (old name lead ethanoate paper black. acetate). Chloride ion (i) If the chloride is soluble, (i) white precipitate of silver add dilute nitric acid and chloride soluble in dilute silver nitrate solution. The ammonia. Cl silver nitrate is acidified with dilute nitric acid to prevent the (ii) You get nasty If the solution also contains the precipitation of other nonfumes of hydrogen sulphate ion, you test with barium halide silver salts. chloride which turn ions 1st, filter off any barium bluelitmus red and give sulphate precipitate and then test (ii) If insoluble salt, add conc. a white precipitate with for chloride ion. This is because silver nitrate solution. silver sulphate is also ~insoluble, sulphuric acid, warm if necessary then test gas as so the two precipitates of silver (iii) A white ppt. of lead(II) sulfate and silver chloride could for HCl. not be distinguished chloride is formed. (iii) Add lead(II) nitrate (i) Any sulphite salt + hydrochloric acid ==> chloride salt + sulphur dioxide. (ii) The sulphur dioxide reduces the dichromate(VI) to chromium(III). Note: sulphites do not give ppt. with acidified barium chloride/nitrate because sulphites dissolve in acids. (iii) Ba2+(aq) + SO32-(aq) ==> BaSO3(s) BaSO3(s) + 2HCl(aq) ==> BaCl2(aq) + H2O(l) + SO2(aq) (i) Pb2+(aq) + S2-(aq) => PbS(s) (ii) MS(s) + 2H+(aq) => M2+(aq) + H2S(g) (e.g. M = Pb, Fe, Cu, Ni etc.) Then reaction (i) above occurs on the lead(II) ethanoate paper (old name lead acetate). (i) Ag+(aq) + Cl-(aq) ==> AgCl(s) Any soluble silver salt + any soluble chloride gives a white silver chloride precipitate, that darkens in light. (ii) Cl-(s) + H2SO4(l) ==> HSO4-(s) + HCl(g) , then Ag+(aq) + Cl-(aq) ==> AgCl(s) (iii) Pb2+(aq) + 2Cl-(aq) ==> PbCl2(s) solution. Not a very specific test - test (i) is best. Bromide ion Br- Fluoride Ion FFluoride and hydrogen fluoride gas are harmful, irritating and corrosive substances. Iodide ion I- (i) Cream precipitate of (i) Ag+(aq) + Br-(aq) ==> AgBr(s) silver bromide, only soluble in concentrated ammonia. Any soluble silver salt + any soluble bromide gives a cream silver bromide precipitate. (ii) Orange vapour of bromine (ii) The bromide ion is oxidised to bromine and the sulphuric and pungent fumes of acid is reduced to sulphur dioxide. SO2, test for sulphur (ii) If insoluble salt, add conc. dioxide. (iii) Pb2+(aq) + 2Br-(aq) ==> PbBr2(s) sulphuric acid, warm if necessary. (iii) A white ppt. of lead(II) bromide is formed. (iii) Add lead(II) nitrate solution. Not a very specific test - test (i) is best. (i) If the suspected fluoride is (i) There is NO precipitate! (i) Silver fluoride, AgF, is moderately soluble so this test soluble add dilute nitric acid proves little except that it isn't chloride, bromide and iodide! and silver nitrate solution. (ii) Look for etching effects on the surface of the (ii) Hydrogen fluoride gas is produced by displacement (ii) You can warm a solid glass rod. fluoride with conc. sulphuric F- + H2SO4 ==> HSO4- + HF which reacts with the glass silica acid and hold in the fumes to form silicic acid, silicon oxyfluoride, silicon fluoride. The (ONLY!) a glass rod with a chemistry is messy and complex BUT the glass rod is drop of water on the end. clearly etched. (i) If iodide soluble, add dilute (i) Yellow precipitate of (i) Ag+(aq) + I-(aq) ==> AgI(s) , any soluble silver salt + any nitric acid and silver nitrate silver iodide insoluble in soluble iodide ==> silver iodide precipitate, solution. The silver nitrate is concentrated ammonia. acidified with dilute nitric acid (ii) iodide ion is oxidised to iodine and the sulphuric acid is to prevent the precipitation of (ii) purple vapour and rotten reduced to 'rotten eggs' smelly hydrogen sulphide, other non-halide silver salts. (i) If bromide soluble, add dilute nitric acid and silver nitrate solution. The silver nitrate is acidified with dilute nitric acid to prevent the precipitation of other nonhalide silver salts. (ii) If insoluble salt can heat with conc. sulphuric acid, (ii) get purple fumes of iodine and very smelly hydrogen sulphide. (iii) If iodide soluble, add lead(II) nitrate solution. Nitrate ion or nitrate(V) (i) Boil the suspected nitrate ion NO3with sodium hydroxide solution and fine aluminium powder (Devarda's Alloy) or aluminium foil. (ii) Add iron(ii) sulphate solution and then conc. sulphuric acid (the 'brown ring' test) egg smell! (iii) insoluble lead(II) iodide formed (iii) Yellow precipitate of lead(II) iodide. Not too definitive -Test (i) best. Pb2+(aq) + 2I-(aq) ==> PbI2(s) (i) the fumes contain (i) The aluminium powder is a powerful reducing agent and converts the nitrate ion, NO3-, into ammonia gas, NH3 ammonia, which turns red litmus blue, seeammonia t (ii) NO complex of iron(II) formed est details (ii) Where the liquids meet a brown ringforms (iii) Nasty brown gas (beware!) of nitrogen (IV) oxide (nitrogen dioxide) (iii) a general thermal decomposition equation for this reaction is 2M(NO3)2(s) ==> 2MO(s) + 4NO2(g) + O2(g) where M = Pb, Zn, Mg, Cu etc. (iii) Strongly heating nitrates of M2+ salts. Nitrite ion or nitrate(III) No simple test to clearly i.d. it, (i) in acid solution it decomposes to give colourless NO gas which rapidly oxidises to ion NO2nasty brown fumes of NO2, (ii) it decolourises (purple ==> colourless) acidified potassium manganate(VII), (iii) it liberates iodine from acidified potassium iodide solution, (iv) forms ammonia with hot Al powder-foil/NaOH(aq) (see nitrate test) and gives 'brown ring' test - see nitrate tests above. Alkali: Hydroxide (i) Litmus or universal (i) It turns litmus blue, (i) A pH meter gives a value of more than 7, the higher the ion i.e. a soluble base indicator or pH meter. variety of colours univ. ind. pH number the stronger the alkali, the higher the OH(alkali) which forms the dark green - violet for weak - concentration, (ii) ammonia gas is evolved: OH- ion in water (note: (ii) Add a little of an strong. to completely identify alkalis (ii) Ammonia released from the salt. ammonium salt. you need to test for the cation (ii) If strongly alkaline e.g. sodium for NaOH etc.) ammonia should be released, NH4+(aq) + OH-(aq) ==> NH3(g) + H2O(l) see ammonia test for rest of details Chromate(VI) ion (i) Add dilute sulphuric acid. CrO42- (yellow) (ii) Add barium chloride/nitrate solution. These tests are not very definitive, but collectively they are (iii) Add lead(II) nitrate a good 'pointer'! solution. (i) The yellow solution (i) CrO42-(aq) + 2H+(aq) ==> Cr2O72-(aq) turns orange as the dichromate(VI) ion is formed. (ii) Ba2+(aq) + CrO42-(aq) ==> BaCrO4(s) (ii) A yellow precipitate of barium chromate(VI) is formed. (iii) A yellow precipitate of lead(II) chromate(VI) is formed. 'lead chromate' (iii) Pb2+(aq) + CrO42-(aq) ==> PbCrO4(s)








