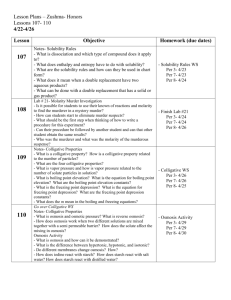
Chemistry * The Science In Context Chapter 8 * Properties of Gases
advertisement

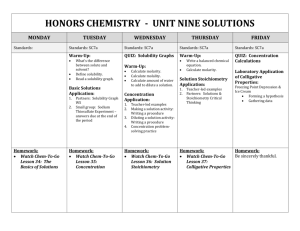
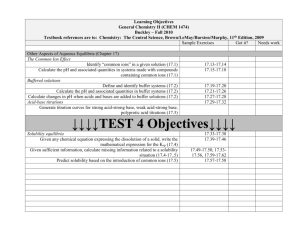
Online Help for Mixtures and Solutions – Chapt 14 Glencoe Matter and Change (Our Textbook) Standardized Test Practice Self Check Quizzes Section 14.1 (Heterogeneous and Homogeneous Mixtures) Section 14.2 (Concentration of Solutions) Section 14.3 (Solvation and Solubility) Section 14.4 (Colligative Properties of Solutions) Education Portal – Chemistry 101 The Rate of Dissolution: Factors and Definition Solutions, Electrolytes and Nonelectrolytes Solubility and Solubility Curves Calculating Dilution of Solutions Georgia Public Broadcasting Video Lectures (~ 30 minute introductory-level videos) Chemistry 1001: Solutions: A Special Type of Mixture Chemistry 1002: Solubility Chemistry 1003: Molarity and Colligative Properties Brightstorm Videos (short videos on specific topics) Types of Solutions Solvation Molarity – Molality Colligative Properties Vapor Pressure Lowering Boiling Point Elevation Freezing Point Depression Colloids - Suspensions Beer's Law Chemguy videos (short videos on specific topics) Junior Chemistry Solutions 1 - describes various solution properties Solutions 2 - describes factors that affect solubility Solutions 3 - describes equilibrium in a saturated solution Solutions 4 - calculates concentration (molarity) and describes solution preparation Solutions 5 - shows the solution preparation apparatus; dilution calculations. AP Chem Solutions 2 - molarity, molality, mass percent and mole fraction Solutions 3 - colligative properties; Tyndal effect; Boiling point elevation; freezing point depression KHAN Academy Videos Khan Academy Chem Site Solubility : Solubility of salt and gas solutes in liquid solvent. YouTube Site Boiling Point Elevation and Freezing Point Supression Suspensions, Colloids and Solutions Learning4Mastery Chemistry Videos AP Chem- Electrolytes-Non-Electrolytes and Solutions (1/2) 14:58 AP Chem- Electrolytes-Non-Electrolytes and Solutions (2/2) 14:58 11.1 (How to calculate in various units of concentration including: molarity, molality, mole fraction, percent solution) AP Chem: Solutions-1: Concentration Units (1/2) 10:56 (11.1) AP Chem: Solutions-1: Concentration Units (2/2) 7:07 AP Chem: Solutions-2: Energetics of solution formation 13:58 (11.2) 11.2 (Vapor Pressure Lowering Boiling Point Elevation Freezing Point Depression How to calculate the molar mass from freezing point data Osmotic Pressure) AP Chem: Solutions-3: Colligative Properties (1/3) 10:01 AP Chem: Solutions-3: Colligative Properties (2/3) 10:36 Can’t find 3/3 PapaPodcasts (High school science/math teacher Toronto, Ontario, Canada) Types of Solutions: Variable Composition and Solubility Types of Solutions: Solubility and Saturation Part 2 Dissociation of Ions in Aqueous Solutions General Solubility Guidelines Solubility Curve and Dissociation Three Factors Affecting Dissolving Part 1 Factors Affecting Dissolving Dipole Attractions Part 2 Factors Affecting Solubility Polar and Non Polar Part 3 Three Factors Affecting Solubility Part 4 Temperature and Solubility of Solids Part 5 Preparing Solutions Introduction Preparing Solutions Sample Problem 1 Preparing Solutions Sample Problem 2 Preparing Solutions Sample Problem 3 Concentration of Solutions Introduction: Mass/Volume... Concentration of Solutions: mass/volume % (m/v)% Concentration of Solutions: Mass/Mass % (m/m)% Concentration of Solutions: Volume/Volume % (v/v) Concentration of Solutions: PPM and PPB Parts Per M/B Molarity/Molar Concentrations Concentration/Molarity W.W. Norton & Company, Chemistry – The Science In Context, 2d edition, Gilbert et. al. Chapter 4: Solution Chemistry and the Hydrosphere Diagnostic quiz ChemTours (basically multi-media tutorials) Molarity Dilutions Migration of Ions Saturated Solutions Chapter 10: Forces Between Ions and Molecules and Colligative Properties Diagnostic quiz ChemTours (basically multi-media tutorials) Henry’s law Boiling and Freezing Points (colligative properties) Osmotic Pressure Raoult's Law (AP level topic) Fundamentals of Chemistry: textbook and self check tutorials Solutions (textbook type material) Continue from the link above using the “next” link on the page to access the textbook topics on the characteristics of solutions, solubility, concentrations, calculations, titrations, ionic reactions, and physical properties. TUTORIALS Molarity Dilutions General Chemistry 1 and 2 – Professor Chuck Wight – University of Utah A series of ten-minute (YouTube) videos that present concepts typically covered in the first and second semesters of a college-level General Chemistry Course. Playlists for Chemistry 1 and Chemistry 2 The video of specific interest is: Liquid Solutions General Chemistry: An Integrated Approach, Third Edition, by Hill and Petrucci Chapter 12: Physical Properties of Solutions The link above will bring you to a page that has links to three different quizzes (Quiz 1, Quiz 2 and Master Quiz). Some e-Media Activities Dissolution of NaCl in Water Enthalpy Diagram Henry's Law Solution Concentration Enthalpy of Solution Solution Formation From a Solid Dissolution of KMnO4 Equilibrium in Solution Formation Equilibrium Vapor Pressure Boiling-Point Elevation and Freezing-Point Depression Semipermeable Membrane Boiling-Point Elevation and Freezing-Point Depression Part II Chemteam - A Tutorial for High School Chemistry The ChemTeam provides study resources in all standard topics for students in high school and Advanced Placement chemistry. Solutions, Concentration, and Colligative Properties A screen shot of the resources on this page appears below.