Heading 1

Comparison of the Ability of Sunscreens to Block UV light
Karen Trickett, Science Dept, Atlee High School, Mechanicsville VA
Cathleen McCarthy-Burke, Biotechnology, Chesterfield Technical Center,
Chesterfield VA
Developed with funding from the Mathematics & Science Center
Time
Question
Grade/Subject
Objectives
Is it possible to receive the same UV protection from sunscreens that contain nanoparticles and those that do not.
6 – 12; Biology, AP Bio, IB Bio, Biotechnology, Ecology, Environmental Science
Discuss 4 features of poor and good scientific sources.
Compare the wavelength and energy of the major regions of the electromagnetic light spectrum.
Describe SPF and what factors contribute the protective rating.
Describe the use of nanotechnology in modern sunscreens.
Design and conduct an experiment to test the efficacy of sunscreens regarding their ability to block UV light.
Evaluate the use of nanoparticle containing sunscreens with respect to risk vs. benefit to individuals and the environment.
Anticipatory Set
Activity 1: Good vs. Poor Sources
Activity 2: The Energy of Light
Activity 3: Bracelet Making with UV sensitive beads
Activity 4: Experimental Design Diagram
Activity 5: Comparison of the Ability of Sunscreens to Block UV light
Experiment
Activity 6: Risk vs. Benefit of Nanoparticle Containing Sunscreens
Activity 7: Debate: Are nanoparticle containing sunscreens the most safe and effective way to protect yourself from the effects of UV radiation?
Closure
Practice
Assessments
10 min
45 min
45 min
30 min
30 min
60 min
45 min
30 min
10 min
Variable
Variable
Materials Activity 1:
For the class:
Class set of copies of the “good” and “poor” scientific sources
1. Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2636591/
2. Your Makeup Could Have an Ugly Effect on Your Health While scientists investigate, make the right choices to avoid teensy-tiny toxins.
http://www.rodale.com/print/587
For each student:
Table to complete for “good” and “poor” scientific sources
Activity 2:
For the teacher:
Background Notes
Completed Guided Note Sheet
For the class:
The Energy of Light – Powerpoint presentation (Slides 1 – 20)
Guided Note Sheet
Activity 3:
For each student:
30 cm of thick hemp jewelry string
20 UV Beads
Stopwatch
Activity 4:
For the teacher:
Completed Experimental Design Sheet
For each student:
Lab Protocol
Experimental Design Sheet
Activity 5:
For each group of students:
1, 4 quadrant divided petri dish
4 3cc syringes
Zinc Oxide
Hawaiian Tropic Sunscreen SPF 60 (chemical based)
Neutrogena Pure & Free Baby Sunblock SPF 60+ (nanomaterial based)
60 UV Beads
Vinyl or Latex Gloves
Stopwatch
Goggles
Activity 6: Risk vs. Benefit of Nanoparticle Containing Sunscreens
For the teacher:
Background Notes
Completed Guided Note Sheet
For the class:
The Energy of Light – Powerpoint presentation (Slides 21 – 31)
Guided Note Sheet
State and National
Correlations
21 st Century
Curriculum
Instructional
Strategies
Virginia Standards of Learning: Science
National Science Education Standards:
Bio 1.b,c,e and h
Nanotechnology: Size and Scale; Size Dependent Properties; Nanotechnology and
Society
1.
Anticipatory Set
1.1 Ask students the following questions:
How many of you lay out in the sun in the summertime?
How many of you work outside?
How many of you go to tanning beds?
How many of you have ever had a sun burn?
1.2. Follow up with these questions:
How could you “protect” yourself from exposure to the sun?
Try to get answers about sunscreen, clothing, sunglasses
Is there such a thing as a “safe tan”?
Why do you tan? Burn?
2 . Activity 1: Learning about Good vs Poor Scientific Sources with articles regarding nanotechnology
2.1. Hand students out 2 articles.
2.2 Ask students to skim the articles, and make some comments about the article itself.
2.3. Ask the students if they believe what they read in both articles and why.
2.4 Tell the students that one article is a poor scientific source, and one article is a good example of a scientific source.
2.5 Ask the students to draw the following table:
Good Source Poor Source
2.6 Ask the students if they can come up with some differences between the 2 articles and complete the table.
2.7 Review student results and complete a table by correcting any misconceptions and filling in any deficits.
3. Activity 2: The Energy of Light
3.1. Students will practice note-taking skills with a guided note sheet while viewing a powerpoint presentation. Pertinent background information will include; the spectrum of light, make-up of skin, sunscreen components and SPF factors.
3.2. Powerpoint Presentation
3.3 Guided Reading Note sheet
4. Activity 3: Making UV Bead Bracelets
4.1. Read procedure
4.2. Make bracelets
5. Activity 4: Complete an Experimental Design Diagram
5.1 Give students the lab instructions for The effect of various sunscreens on their ability to block UV radiation
5.2 After reading the lab instructions, have the students complete the Experimental
Design Worksheet
5.3 Review the Experimental Design Worksheet with students
5.4 Break into lab groups, assign roles of reader/leader; materials manager, data recorder; technician
6. Activity 5: The Effect of various sunscreens on their ability to block UV radiation
6.1. Complete Lab
6.2. Complete Results Table, and Conclusions Questions
7.
Activity 6: Risk vs. Benefit of Nanoparticle Containing Sunscreens
7.1
Students will practice note-taking skills with a guided note sheet while viewing a powerpoint presentation. Pertinent background information will include; safety concerns to humans and the environment; and as well as labeling practices of products containing nanoparticles
7.2
Powerpoint Presentation
7.3
Guided Note Sheet
8. Activity 7: Debate
8.1 Are nanoparticle containing sunscreens the most safe and effective way to protect yourself from the effects of UV radiation?
8.2 Divide class into 3 sections; one for yes, one for no, one for moderators
8.3 Using their readings, notes, and results from the experiment, Yes and No students will prepare their arguments. The moderators will develop follow-up questions that they would like to present to both teams.
8.4 Allow each side 10 mins to present their case, followed by 5 mins of questioning for each team.
8.5 Moderators will then vote to determine whether nanoparticle containing sunscreens are the most safe and effective way to protect humans from UV radiation.
Closure
Extensions
Assessment
Describe to students how through this unit, they have modeled the scientific method in investigating a new topic.
Everything begins with an observation, and asking a question – Anticipatory
Set
Background Research – Activities 1, 2, 3,6
Designing an experiment to answer your question – Activities 4,5
Sharing your results with others – Activity 7
1.
Title
Materials:
Directions:
2.
Title
Materials:
Directions:
Sample items are provided for use in checking students’ understanding.
Paper Pencil Test: Written Unit Test
Product: Lab Report
Persuasive Essay for use in debate
The following table shows how the assessment items are related to specific objectives.
Describe SPF and what factors contribute the protective rating.
Describe the use of nanotechnology in modern sunscreens.
Design an experiment to test the efficacy of sunscreens regarding their ability to block UV light.
Objective
Evaluate the use of nanoparticle containing sunscreens with respect to risk vs. benefit to individuals and the environment.
Paper-Pencil
Test
Product/
Performance
Students will describe 4 features of a poor and good scientific source.
Compare the wavelength and energy of the major regions of the electromagnetic light spectrum.
Questions on
Unit test.
After reading articles, complete table.
Questions on
Unit test.
Questions on
Unit test.
Pre-lab experimental design diagram.
Completed lab report.
Student led debate.
Teaching
Tips
1.
Some tips about the materials used in this lesson:
Different colors of UV beads are available.
Any type of sunscreens can be used, whatever is available in your area.
Gloves should be worn while working with the sunscreens because of possible allergic reactions, and also for protection against any drawbacks of the nanomaterials.
2.
Where do we get the supplies : (provide links)
A. Educational Innovations, Inc
362 Main Avenue
Norwalk, CT 06851
888-912-7474 www.teachersource.com
UV Beads –
UV-RED
2 oz. pkg. [~240 beads per pkg.]
1 or more: $6.95
ea
B. Wards Natural Science
PO Box 92912
Rochester, NY 14692-9012
800 – 962-2660
4 Quadrant Petri Dishes 18W 7108 20 plates/$5.95
3cc syringes 14W 1615 10/$6.00
C.
Rite Aid
Zinc Oxide
Hawaiian Tropic Ozone Sport 60+ sunblock (Chemical based Sunscreen)
D. Babies R’ Us
Neutrogena Pure & Free Baby Sunblock SPF 60+. ( Nanomaterial based Sunscreen)
3.
What are the answers to the Paper/Pencil test?
Table of Good vs. Poor Sources
Good Source
Authors, from research facilities
Article is from a journal
.edu, .gov
Article contains features of the scientific method: controlled experiment, data, graphs, conclusions
Journal article has citations
Journal articles are peer reviewed
Poor Source
Author is not an authority
Article is from a magazine, paper
.com
Article does not have experimental data, pictures may general
No citations present
If any review, it is an editor
References MathScience Innovation Center
Information on educational programs available to students, teachers and school divisions and procedures for registering for programs. http://msinnovation.info
Mathematics & Science Center: On-Line Educational Programs
Learn through on-line virtual classrooms, web-based lessons and on-line courses.
Access proven lesson plans and instructional modules. http://www.mathinscience.info
Educational Resources, Ultraviolet Radiation http://www.nas.nasa.gov/About/Education/Ozone/radiation.html
Appendix 1: Good vs Poor Sources
Good Source
Good Source
Authors, from research facilities
Article is from a journal
.edu, .gov
Article contains features of the scientific method: controlled experiment, data, graphs, conclusions
Journal article has citations
Journal articles are peer reviewed
Poor Source
Poor Source
Author is not an authority
Article is from a magazine, paper
.com
Article does not have experimental data, pictures may general
No citations present
If any review, it is an editor
Appendix 2: Teacher Background Notes
Teacher Background Information
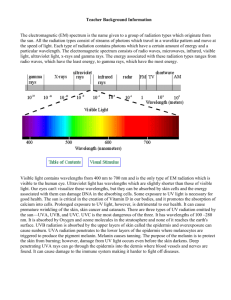
The electromagnetic (EM) spectrum is the name given to a group of radiation types which originate from the sun. All the radiation types consist of streams of photons which travel in a wavelike pattern and move at the speed of light. Each type of radiation contains photons which have a certain amount of energy and a particular wavelength. The electromagnetic spectrum consists of radiowaves, microwaves, infrared, visible light, ultraviolet light, x-rays and gamma rays. The energy associated with these radiation types ranges from radio waves which have the least energy to gamma rays which have the most energy.
Visible light contains wavelenths from 400 nm to 700 nm and is the only type of EM radiation which is visible to the human eye. Ultraviolet light has wavelengths which are slightly shorter than those of visible light. Our eyes can't visualize these wavelengths but they can be absorbed by skin cells and the energy associated with them can damage DNA in the absorbing cells. Some exposure to UV light is necessary for good health. The sun is critical in the creation of Vitamin D in our bodies, and it promotes the absorption of calcium into cells. Prolonged exposure to UV light, however, is detrimental to our health. It can cause premature wrinkling of the skin, skin cancer and cataracts. There are 3 types of UV radiation emitted by the sun --UVA, UVB, and UVC. UVC is the most dangerous of the three. It has wavelengths of 100 - 280 nm.
It is absorbed by Oxygen and ozone molecules in the stratosphere and none of it reaches the earth's surface.
UVB radiation is absorbed by the upper layers of skin called the epidermis and overexposure can cause sunburn. UVA radiation penetrates to the lower layers of the epidermis where melanocytes are triggered to produce the pigment melanin. Melanin causes tanning. The purpose of the melanin is to protect the skin from burning, however, damage from UV light occurs even before the skin darkens. Deep penetrating UVA rays can go through the epidermis into the dermis where blood vessels and nerves are found. It can cause damage to the immune system making it harder to fight off diseases.
UV radiation which reaches the Earth has become more abundant over the last century and continues to increase. This is due to the thinning of the stratospheric ozone layer. Chloroflourocarbons
(CFC's) have been implicated in the majority of ozone damage. The CFC molecules lose Chlorine atoms from their structure which attack and destroy ozone molecules in the stratosphere. With large numbers of ozone molecules destroyed, thinning of the ozone layer occurs which lowers its effectiveness in filtering out harmful UV rays. Since UVA light does not penetrate very far into the skin, the damage it does to the DNA of cells has been linked to skin cancers which appear in the upper layers of the skin. These skin cancers include basal cell carcinomas which are the most common of all skin cancers and squamous cell carcinomas which are the 2nd most common. Eight hundred thousand basal and squamous cell carcinomas are diagnosed annually in the US. UVA damage to the dermis is also the main cause of premature aging of the skin. Deeper penetrating UVB rays can lead to the least common but most deadly form of skin cancer -- malignant melanoma. These are cancers related to the melanin producing cells deep in your skin layers. If not found and treated early, it can quickly spread from the skin to other body organs. More than 1 million cases of skin cancer are diagnosed in the US annually. Higher numbers of younger people are diagnosed with melanoma than ever before. A 1% decrease in the ozone layer is estimated to cause a 2% increase in
UVB radiation reaching the earth which can lead to a 4% increase in basal cell carcinomas.
To prevent the damage caused by both UVA and UVB radiation, sunscreens or sunblocks are recommended. The first commercial sunscreens became available during WWII. At this time, US sailors used zinc oxide to prevent sunburn. Sunscreens eventually became available to the general public in the
1950's and 1960's. Today, 17 sunscreen active ingredients are approved for use in the US. Twenty five are approved in Europe. Sunscreens combine organic and inorganic active ingredients to either absorb, reflect or scatter the sun's rays on the skin. Inorganic active ingredients include Zinc Oxide (ZnO) or Titanium
Oxide (TiO
2
). They act as a physical block to UV rays and reflect or scatter them off the skin. Organic active ingredients include octyl methoxycinnamate or oxybenzone which absorb UV radiation, dissipating it as heat out into the environment. Different ingredients vary in the type of radiation they are best at protecting against. The majority of sunscreens on the market are specialized to block UVB rays (290 - 320 nm). These sunscreens include those that contain one or more of the following ingredients: padimate O, homosalate, octylmethoxycinnamate, benzophenone, octyl salicylate, phenylbenzimidazole, sulfonic acid
and octocyclene. Since no single chemical blocks the full range of UVB wavelengths, a combination of up to 3 of these chemicals are typically used to provide wider coverage. Recently, more broad spectrum sunscreens have become available which retain their UVB protection and add protection for UVA rays as well (320 - 400 nm). This would include adding oxybenzone (for shorter UVA wavelengths) or avobenzone (for longer UVA wavelengths) to the formula. Alternatively, physical sunblocks which contain either TiO
2
or ZnO reflect both UVA and UVB rays thus providing broad spectrum protection. Since they protect over the entire range of UV wavelengths, they can be the sole ingredient in the sunscreen.
Traditionally, sunbathers have shied away from the inorganic mineral sunblocks containing zinc or titanium oxides due to their being an opaque white color instead of transparent. Recently, however, zinc oxide and titanium oxide have been produced on the nanoscale with particle sizes of billionths of a meter.
This is much smaller than the usual particle size of the minerals and as a result of their smaller size are transparent instead of opaque white. Sunscreen manufacturers have used nanosized Titanium dioxide since
1990 and nanoscale zinc since 1999. Today there are over 300 sunscreens on the market which contain these nano-sized particles. The ingredients are extremely effective at deflecting the sun's rays off the skin, therefore preventing penetration of the UV rays which not only prevents burning, but also any damage to genetic material of the cells which could lead to cancer. Some studies have shown that consumers who use sunscreen products that do not contain zinc or titanium are exposed to 20% more UV radiation than those who do. While, zinc and titanium nanoparticles are effective at preventing sunburns and associated sun damage, there is controversy about the use of these particles in sunscreens. The controversy stems from the lack of definitive safety data and consumer information on sunscreens that contain these particles as well as the lack of requirements to label sunscreens as containing nano-sized particles. Some concerns are that the nanoparticles could be absorbed through the skin of the user into the lower, viable layers of skin cells and then react in a way which could be unsafe inside the human body when exposed to sunlight. Nanosized particles are well known to be more reactive than their larger counterparts. These particles are known to emit free radicals upon absorption of UV or visible light which could cause damage to the biomolecules such as DNA inside the cells. In addition, many are concerned about the particles washing off the skin and entering the environment where they could cause unknown damage.
To answer some of the concerns regarding nanoparticles in sunscreens, the Environmental Working
Group conducted a study of the available scientific literature including 400 peer reviewed studies on the topic of nanoparticles in sunscreens. They concluded from their review that sunscreens without zinc and titanium actually have four times as many hazardous ingredients. Some of the ingredients in traditional sunscreens are suspected to cause cancer or birth defects and have been shown to mimic estrogen in the body which may have a potential breast cancer link. In addition, 16 peer reviewed studies on the skin absorption of zinc and titanium nanoparticles, showed little or no absorption though healthy skin. On the other hand, octinoxate and oxybenzone (chemicals found in traditional sunscreens) have been shown to be absorbed through healthy skin and also to cause allergic reactions in some. As a result, the Environmental
Working Group concluded that zinc and titanium formulas are probably the safest and most effective products on the market based on available evidence. This is an interesting endorsement as the EWG conducted the review with the idea that they would not be recommending these products. They, however, only recommend them in lotion form and not in spray or powder form as these types may cause inhalation of these nanosized particles which can produce an inflammatory response due to the high surface area of the particles involved. In 2000, the European Union approved nanosized titanium for use in sunscreen and concluded that it does not penetrate the skin and presents no risk of cytotoxicity, phototoxicity or genotoxicity. Nanosized zinc was not approved by the European Union due to the fact that not enough research could be found on its safety. In 2009, the Therapeutic Goods Administration (TGA) in Australia conducted an updated review of scientific literature on the topic of absorption of nanoparticles from sunscreen. It concluded that the weight of current evidence suggests these particles do not reach viable cells
but instead remain on the surface of the skin which is composed of non-living cells. In the US, the FDA has not conducted any studies about the safety of these chemicals at the nano- scale.
An additional concern about these sunscreen ingredients is their environmental effect when they wash off skin into water and then enter reservoirs or natural ecosystems. Both zinc and titanium are known to have strong antibacterial properties. These antibacterial properties could be magnified with nano-sized particles since they have smaller surface area and are more reactive. In a study which appears in Scientific
American in March of 2009 which was done at the University of Toledo, it was concluded that nanosized titanium dioxide molecules reduced the effectiveness of environmental bacteria after less than 24 hours of exposure. They added varying amount of nanoparticles to water which contained bacteria and found that the bacteria had significant cell wall damage when the amounts were as small as 10 - 100 mg/L of the nano
TiO2. In anther study done at Utah State University and the University of Utah, beneficial soil bacteria were shown to be damaged by nanoparticles of silver, copper oxide and zinc oxide even at very low levels of exposure. Additional concern comes from the unknown unintended effects of these particles on the environment. Clearly, more research needs to be conducted before the environmental safety of sunscreen products can be ascertained.
Appendix 3: SPF to SNF Powerpoint
SENT AS A SEPARATE ATTACHMENT
WITH THIS FILE
Appendix 4: Guided Note Sheet
From SPF to SNF
(Sunscreen Nano Facts)
Note taking Guide to Accompany Powerpoint Presentation
Part 1: The Energy of Light
The Electromagnetic spectrum consists of 7 types of radiation each with a different wavelength and energy level:
1) _________________________ 2) ________________________
3) _________________________4) ________________________
5) _________________________6)_________________________
7) ______________________________
The rays with the shortest wavelengths are ________________ and with the
Longest wavelengths are ___________________.
Ultraviolet light has wavelengths of between _______ and ______ nanometers (nm). The are
________________ to the human eye but are ____________________ by skin cells and can damage ______________.
The 3 types of UV light are:
1) __________________ (100 – 280 nm)
2) __________________( 320 – 290 nm)
3) __________________ ( 320 – 400 nm)
Some UV light is needed to ____________________ and ____________________.
Absorption of UVB light by skin causes ___________________.
Absorption of UVA light causes __________________ to produce
________________ which results in a __________.
The UV rays that allow tanning cause the following types of skin damage:
_________________________, ___________________________, and ___________________________________________.
The 3 types of skin cancer are
1) the most common type is ____________________________________
2) the 2 nd most common type is __________________________________
3) the rarest but most dangerous type is ____________________________
To protect against UV rays _________________ and _________________ are used. The active ingredients in sunscreens either __________________,
____________________ or ______________________ UV rays.
The active ingredients in sunscreens are either
1) inorganic - such as _________ and __________ which scatter rays off skin
2) organic - such as _________________________ or
______________________ which absorb UV light and disperse
it as heat.
Broad spectrum sunscreens protect against both ______and ______ rays.
A sunscreen’s SPF is a measure of ________________________________
___________________________________________________________________
___________________________________________
Many people don’t use ZnO and TiO2 because ________________________
________________________________________.
Nanotechnology is the science of materials in the range of _______to _______ nanometers.
Nanosized particles often exhibit unique _______________, _____________, and
________________ properties.
A nanometer is a ________________ of a meter.
As nanoparticles, ZnO and TiO2 are ______________________________.
Part 2: Risks Vs. Benefits
2 safety concerns exist regarding the use of nanoparticles in sunscreens. They are:
1) _____________________________________________________
_____________________________________________________
_____________________________________________________
2) _____________________________________________________
_____________________________________________________
_____________________________________________________
Many feel that __________________ of sunscreens that contain nanoparticles should be mandatory. However, so far in the US the
________________ does not require these labels.
Appendix 5: Procedure for making UV bead bracelets
UV Bead Bracelet Activity
Objective: The students will create a bracelet made of UV beads and expose it to UV light outside the classroom. Through this activity, students will gain an understanding of how the UV beads react when exposed to sunlight for different time periods and create a 0 – 3 qualitative scale of color.
Materials Needed:
30 cm. piece of pre-cut hemp cord
10 UV beads
Stopwatch
Colored pencils
Lab directions
It is important to choose a sunny day to complete this activity
Directions:
1) Break students up into groups of 4. Each group should needs a copy of the lab directions, a stopwatch, and colored pencils in the color that the UV beads will change .
2) Each student should get 1 piece of 30 cm. pre-cut hemp cord and 10 UV beads.
3) Each student should tie a knot in their hemp cord about 10 cm. in from one end. Thread the 10 beads onto the thread and arrange them so that they stop at the knot. Tie a second knot at the end of the line of the 10 UV beads. Have a lab partner tie the bracelet onto one of their wrists so that the beads are resting on the top of their wrist.
4) One student should be assigned to expose their bracelet for 0 sec. to UV light, one for 10 sec., the third for 20 sec. and the final student for 30 sec.
5) Once all the students in the group have made their bracelets and have them on their wrists, the group should proceed outside carrying the stopwatch, colored pencils, their own writing instrument and the lab direction sheet with them. One student needs to hold the stopwatch and
Be responsible for timing the 30 second time frame. (It will probably work best if this is the
30 sec. person)
6) Immediately upon reaching sunlight the timer should turn on the stopwatch and begin timing. The student assigned to the 0 sec. of UV light exposure should not allow the beads to be exposed to any sunlight. They can accomplish this by keeping their other hand tightly over the beads.
7) When 10 seconds have elapsed, the timer should call out “10 sec.” and the
Student who was assigned this time frame should cover the beads with their other hand.
Repeat this step for 20 sec. and 30 sec.
8) At the end of 30 seconds, all students should return to the classroom and
note the color of the UV beads in the following table. In addition to noting
the color, they should use the colored pencil to indicate the shade of the
beads to the best of their ability.
Table 1: Results of UV bead exposure to sunlight at various times
Time (sec) Color of beads Shaded drawing of bead
0
Scale =0
10
Scale = 1
20
Scale = 2
30
Scale = 3
Appendix 6:
Experimental Design Diagram
1. Title:
The effect of _______________________________________ (independent variable) on _______________________________________________ (dependent variable)
2. Hypothesis:
If ____________________________________________ (planned change in the I.V.)
Then _________________________________________ (predicted change in the D.V.)
3. Independent Variable: ________________________________________________
4-6. Levels of the Independent Variable, Control Group, # of Repeated Trials
Level 1 (Control
Group)
Level 2 Level 3 Level 4
# of Trials # of Trials # of Trials # of Trials
7. Dependent Variable and how it will be measured
__________________________________________________________________
8. Constants (4)
1.
2.
3.
4.
RESOURCE
• Students and research: Practical strategies for science classrooms and competitions.
Cothron, J. H., Giese, R. N., & Rezba, R. J. (3rd Ed.). (2000). Dubuque, IA:
Kendall/Hunt Publishing Company.
Appendix 7:
Title: The Effect of Sunscreen ingredients on the ability to block UV radiation
Purpose: The purpose of this experiment is to test the ability of sunscreens, containing various active ingredients, on their ability to block UV radiation.
Materials:
1, 4 quadrant divided petri dish
1 mL Zinc Oxide
1 mL BananaBoat Lotion Sunscreen SPF 60 (chemical based sunscreen)
1 mL Water Babies (?) Lotion Sunscreen SPF 60 (nanomaterial containing sunscreen)
60 UV Beads
4, 3cc syringes
Goggles
Vinyl or Latex Gloves
Sharpie pen
Stopwatch, or watch with second hand
Procedure:
1.
Using the sharpie marker, draw 2 lines on the top of the petri dish marking the 4 quadrants.
Write a very small 1, 2, 3 or 4 in each quadrant.
2.
Using the sharpie, label the bottom of the petri dish so that one quadrant is marked 1, 2, 3, or
4.
3.
Place 15 UV beads into each quadrant, and place the lid back onto the dish. Make sure to line up the quadrants with the corresponding numbers on the top and bottom.
4.
Put your gloves on and keep them on until you are done handling the sunscreens.
5.
Suction 1 mL of zinc oxide into a 3cc syringe.
6.
Slowly push the plunger down and release this sunscreen onto the top of the petri dish marked with the #2. Gently, spread the zinc oxide around covering the entire top of quadrant 2 and the side of the dish associated with quadrant 2. This sunscreen will still be thick and white.
7.
Repeat steps 5 and 6, placing BananaBoat sunscreen onto the top and sides of Quadrant 3, and
_________________ sunscreen on the tops and side of Quadrant 4. Try to get these sunscreens to be fairly clear.
8.
Carefully, pick up the petri dish, and rest the bottom, in the palm of your hand.
9.
Carry the dish towards the outside sunshine.
10.
Begin the stopwatch, and place in the sunshine at the same time.
11.
Hold the dish in the sunshine for 2 minutes.
12.
Take the dish to a shady area.
13.
Record your results in the data table, using +++++ for maximum UV absorbance, ++++, +++ ++, +, and – for no detectable absorbance of UV light.
14.
The +++++ will be based on the color of the beads in Quadrant 1, your control.
Results:
Table 1: Color of the UV Beads following exposure to UV light
Quadrant Contents
1 Clear
Color of Beads
2 Zinc Oxide
3
4
SPF 60; octocrylene + oxybenzone + avobenzone
SPF 60; zinc oxide + titanium dioxide
Conclusions:
1.
What was the purpose of including Quadrant 1 in this experiment?
2.
Referring back to your experimental design diagram, write down your hypothesis.
3.
What sunscreen(s) was the best at blocking UV light? How did you know this?
4.
Does the data that you collected support your hypothesis? Why or why not?
5.
What were 2 possible sources of error? Describe what part of your experiment that they may have impacted, and what could be done to avoid this is in a future experiment.
6.
Based on your findings, what future experiment would you like to perform?