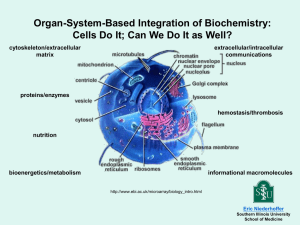
Open Access version via Utrecht University Repository
advertisement

Determination of the passage rate in saltwater crocodiles (C. porosus) using beads, flagging tape and fluorescent pigment as digestive markers Student: Eveline Dijkstra Student number: 3258882 Supervisors: Dr. M.J.L. Kik Dr. C.M. Gienger Utrecht University - Faculty of Veterinary Medicine, July 2014 Abstract The saltwater crocodile (Crocodylus porosus) is extensively farmed for their skin and meat. In order to optimise growth rates and production crocodiles need to be fed an optimal diet, based on their nutritional physiology. In this study two experiments were conducted to determine the passage rate of food in juvenile and sub-adult crocodiles. Plastic beads, pieces of flagging tape and a fluorescent pigment were used as digestive markers, to evaluate whether these markers are suitable as digestive markers in C. porosus. Results showed differences in excretion patterns between the type of markers. Large percentages of beads and pieces of flagging were retained in the stomach, not passing the digestive system. The fluorescent pigment was excreted by juvenile crocodiles almost continuously during the experiment. Despite the fact that the fluorescent pigment did pass the digestive tract, the obtained data is inaccurate. It can be concluded that the used markers are not useable as digestive markers in crocodiles, since they were not successful in determining the passage rate in juvenile and sub-adult C. porosus. More precise markers need to be used as digestive markers in crocodilians to achieve more accurate knowledge about the crocodile digestive system to eventually improve crocodile nutrition. Introduction The saltwater crocodile (Crocodylus porosus) is extensively farmed for their skin and meat1,2. Similar to sectors of extensive animal production systems, nutrition is considered to be of major importance in crocodile farming to optimise growth rates of individuals, and therefore production1,3. In the past few decades many attempts have been done to improve the diet of farmed crocodiles. However, in order to compose an optimal diet for farmed crocodiles it should be based on their nutritional physiology. Surprisingly only little accurate data is known about the digestive physiology of any crocodilian species. As part of a larger study, to provide data about the digestive efficiency of several crocodile species, this study was designed to define the rate for food to pass the gastro-intestinal tract of juvenile and adult saltwater crocodiles. The passage rate defines the rate at which residues of the diet run through the digestive tract, the time from ingestion to defecation4. The passage rate is a commonly measured variable in digestion studies, since it is an estimation of the retention time. The retention time is a term for the period in which the digesta remain in the absorptive regions of the digestive tract. It reflects the gut evacuation and is therefore related to the digestion rate. Consequently the retention time can be an indication of the time and energy required to digest food5. The gut passage rate between species is extremely variable6. Compared to ectotherm species, endotherms have relatively short and consistent intestinal passage times7. The physical performance of ectotherms is greatly influenced by external factors. The passage rate in reptiles is affected by their body temperature8,9, diet quality10, diet quantity9, type of dietary marker8,11, and the morphology of the digestive tract. For example, among the herbivorous reptiles the proximal colon of iguanid, agamid, and scincid species contains transverse valves. The number of these valves is positively correlated with the adult body size, and may as well have impact on the passage rate12,13. Furthermore, just like in humans, simply individual variation is a possible cause for variability, even under controlled conditions6. Most studies used markers to determine the passage rate. Along with the large number of digestive studies on different species, an extensive variety of markers came to existence since not all markers are suitable to the morphology of every digestive tract. Besides, there are some other practical considerations which may decline their usability, for example the type of data that needs to be collected or the environment in which the animal lives. In the next paragraphs several types of markers, used in reptilians over the past decades, are reviewed. This review will give a structured overview of the markers concerning their effectiveness during the trials, their advantages and disadvantages. For a quick overview and comparison of the discussed studies, the data of the reviewed articles are recorded in table 5 (appendix, p. 32). Digestive markers In order to define the gut passage rate, the faeces of the experimental diet needs to be distinguishable from other meals. In most cases a diet is mixed or labelled with a marker that eventually will reappear in the faeces. The rate at which the marker reappears is the rate for undigested residues of the diet to pass the digestive tract4. To ensure the reappearance of the marker, a marker has to meet to certain characteristics. The ideal dietary marker is non-absorbable and has the same particle size and density as the natural diet. This way, the marker will be recoverable qualitatively and quantitatively in the faeces, and will the marker behave similarly to the diet along the digestive tract. Also the passage of digesta should not be influenced by the marker4. There are two options to mark an experimental diet; either by adding an inert marker to the diet, or by feeding a diet that is different in some identifiable way, for example by colour or texture4. In the scenario the marker will be called an external marker, since it is artificially added to the diet. In the second option the identifiable parts of the diet can be defined as internal markers, as the markers are a natural en integral part of the diet. In a study of Greenwald et al. (1979)14 the faeces from a given meal could be identified by the undigested hair of mice which were fed to corn snakes (Elaphe guttata guttata). Sadeghayobi et al. (2011)15 used the seeds of three different types of fruit as a marker to determine the digesta retention time in Galapagos tortoises (Geochelone nigra). By just feeding an animal’s natural diet, without adding a foreign material to the experimental diet, all possible side effects of a marker are eliminated. However, quantification is not possible using this technique4. Most studies used a technique in which they added an external marker to the experimental diet. Based on the literature of digestion studies in reptilians, basically four groups of inert faecal markers can be defined; pigments and dyes, particulate markers, chemical markers, and radiopaque markers. Pigment and dyes The first category ‘pigments and dyes’ includes products which are added to stain the experimental diet. Carmine red is a frequently used marker in humans16, mammals17, fish18,19 and reptiles1,20. Other examples are the vital dye indigo carmine8,21 and fluorescent pigment5,22,23. A food colouring gel was used in a study with Komodo dragons (Varanus komodoensis)24. Food colouring products are often used as a marker since they are easy to obtain and are generally safe to use. All of these studies used their marker to determine the time between the food intake and the first appearance of the coloured diet in the faeces. Some of them also defined the last appearance in the faeces1,5,20. Because of the small particle sizes, stains and dyes will easily mix with the diet, causing no mechanical disruption of the passage of the food along the gut4. According to Zimmerman and Tracy (1989)4 the use of a stain or a dye is not a quantifiable technique, since they are prone to partial absorption. Also secondary staining of food material inside the gastrointestinal tract makes the interpretation of the staining technique complicated8. This could be an explanation for the wide range between the first and last appearance of the markers in the studies by Garnett (1988)1 and Liesegang et al. (2001)20. Even though Hatch and Afik (1999)5 used a spectrophotometer to determine the percentage of fluorescent marker present in each faecal sample, most findings are merely based on macroscopic visual detection. Therefore data concerning the appearance of these markers in the faeces should be carefully interpreted. In addition, not every stain or dye sticks to the same fraction of the diet. Beaupre et al. (1993)23 and Hatch and Afik (1999)5 observed a difference in transit time between a water soluble dye, such as carmine red25, and a fluorescent powder. The water soluble dye appeared in the faeces long before the fluorescent powder. From this they concluded that the soluble dye stained the liquid phase of the diet and the fluorescent powder the solid phase. However, no statistical results are presented in the study of Beaupre et al. (1993)23 and the study of Hatch and Afik (1999)5 reported no significant differences in transit times between the used markers. It is argued that the difference in transit time for each phase depends on the ability of a certain species to separate the liquid from the solid phases in the gut5,23. These results point out another difficulty of comparing gut transit times among several studies using different markers. Particulate markers The following group of inert markers are the particulate markers. These markers are defined as synthetic indigestible, non-absorbable organic substances, which are clearly recognizable in faeces. Particulate markers can be subdivided in non-quantifiable and quantifiable markers. Examples of non-quantifiable markers are glitter and fluorescent polyacrylamide beads. Both markers are generally larger in diameter than pigments and dyes, but still too small to use them as quantifiable markers. These markers are mostly used for the same purpose as stains and dyes. Because of their considerably small particle size they still easily blend with the experimental diet. Kummrow et al. (2011)26 used crickets coated in glitter (< 1 mm) as a digestive marker in chameleons (Chamaleleo calyptratus). Faecal samples were visually examined for the excretion of glitter. Fluorescent polycrylamide beads, used by Waldschmidt et al. (1986)9, are beads of 100 μm in diameter which are chemically bonded to a fluorescent dye. They used this marker in the insectivorous side-blotched lizard (Uta stansburiana) and were able to detect the beads in the faeces by examination with a UV microscope. Zimmerman and Tracy (1989)4 used fluorescent polyacrylamide beads to determine the passage rate in chuckwallas (Sauromalus obesus), but weren’t able to detect the markers in the faeces. They discussed that this could be either because the beads never appeared in the faeces, perhaps because they retained in the deep colic valves, or the beads no longer fluoresced after passing through the digestive tract. The first reason would imply that the beads aren’t immiscible with the food, which would affect the passage time results. Quantifiable particulate markers are frequently used as digestive markers in studies with reptiles, especially in field studies since they are easily identifiable. Beads and pieces of plastic tape are the most commonly used markers in this category. However, because beads and plastic pieces have a relatively large particle size, these markers are immiscible with the food. Therefore reported transit times in studies using these types of markers actually measured the passage rate of the marker itself. In a study of Harwood (1979)8 two markers were used; carmine red and pieces of vinyl tape. The vinyl tape usually passed through the digestive tract more slowly than the stained food. He states that this increase in residency could have been caused by temporary adhesion of the vinyl tape to the intestinal mucosa. A study of Troyer (1984)27 showed that glass beads of 2 mm in diameter and 4 mm discs of vinyl flagging tape moved at the same rate in green iguanas (Iguana iguana). She also noticed that the initial experimental diet and its markers appeared together in the faeces, what would assume that the markers moved at the same rate as the diet. Wikelski et al. (1993)28 found similar results in a study with marine iguanas (Amblyrhynchus christatus). Valente et al. (2008)29 used very-low-dense coloured dishes of flat foam (5 mm in diameter), which appeared at a faster rate in the faeces than a simultaneously used marker with a higher density. They assumed that the colour markers travelled with the fluid-phase of the digesta. In a study of Hailey (1998)30 a combination of loops of polyester thread (2cm in circumference) and radiopaque Ballotini microbeads (0,65-0,75mm) was used to determine the passage rate. The recovery of beads was higher than the number of loops found in all cases. He states that this might be due to fact that fibrous material passes more slowly through the gut than particulate material such as microbeads. Additionally, the size of the marker might influence the passage rate. In a study of Spencer et al. (1998)31 the 25mm2 plastic pieces had a longer transit time than the 1mm2 pieces of plastic. Since these type of markers are quantifiable, other options for defining the transit time are possible. Just like with a pigment or a dye the transit time can be defined as the time between feeding and the first appearance of a marker, the minimum transit time31,32. This definition is generally used in studies using a single particulate marker. In experiments using a multiple number of markers the maximum transit time (the time till last the appearance of the marker)15, the modal transit time (time till the day on which the maximum number of markers reappear), and the median transit time (time till 50% of the markers is excreted) can be determined30. Others defined the gut transit time as the time between feeding and the time a certain percentage of the marker has appeared in the faeces. A minimum of 50% is frequently used27,33. Amarocho and Reina (2008)34 defined the total passage time as the time at which at least 73% of the markers was recovered and Valente et al. (2008)29 at 85%. Often this terminology misused or given a different interpretation. For example, tmax is used as the time till last appearance of the marker but also as the time at which the maximum concentration of marker appeared10. Chemical markers In order to find the most accurate marker, researchers developed techniques using chemicals as a digestive marker. Several chemicals can be used to bind specific elements in the feed and their concentrations can easily be measured in the faeces. Chromium is by far the most frequently used chemical marker in animal digestion studies3,10,35-37. However, the use of chromic oxide is often criticised because it does not associate specifically with either the particulate or the fluid phase of the digesta35. Also, dietary chromic oxide is not always totally recovered from the faeces and therefore it needs to be used at relatively high concentrations, which may affect the absorption and metabolism of nutrients35,36,38. Mixing a diet with chromic oxide will colour the feed and eventually the faeces green. This way in some studies it is used as pigment36,37. On the other hand, the alteration in colour of the feed may influence the voluntary ingestion. Finally, the use of chromic oxide may be a health hazard, since it may be toxic even at low concentrations35,38. In order to nip problems concerned with marking the right components of the digesta, a dual-phase marker technique is developed. This is a technique in which several markers are used simultaneously to mark the fluid-phase and the solid-phase of the faeces35. Polyethylene glycol, Co-EDTA and CrEDTA are frequently used fluid-phase markers, while chromium and rare earth elements are commonly used as particulate-phase markers35,39. The dual-phase marker technique is frequently used in studies concerning herbivorous species, since especially rare earth elements have a strong affinity for plant cell walls and can be used in their soluble form to label indigestible plant fibre40. Despite these characteristics, recently a couple of studies is published using this technique successfully in carnivorous mammals41-43. Apparently the binding capacity of rare earth elements is related to the cation exchange capacity of the feedstuffs to which labelling takes place. Carboxyl, hydroxyl and amino acid nitrogen groups seem to be the functional groups concerning the rare elements binding39. These markers can be measured in feed and faeces at low concentrations in the mg kg-1 range. Because of the accuracy of these markers and the high percentages of recovery, these markers are commonly used to determine the mean retention time of various components in the feed and to estimate the apparent digestibility of nutrients in the feed. Barboza (1995)10, Hatch and Afik (1999)5, and Tracy et al. (2006)44 performed this technique in their studies, using a herbivorous or omnivorous reptile species. They were able to determine the flow rate of the liquid-phase and the particulate-phase of the digesta. In all studies the liquid-phase appeared before the particulate-phase. Also different flow rates were found for particles with various lengths. The larger the particle, the longer the retention time. In the study of Hatch and Afik (1999)5 polyethylene glycol was used as an aqueous marker and glycerol triether as a lipid-marker. A lack of significant difference was found between the lipid marker and the aqueous marker. They also did not find a significant difference between these markers and the fluorescent powder marker they used, making the fluorescent powder a plausible marker for reptiles who don’t measurably separate the liquid from the solid phases in the intestinal tract. Radiopaque markers Radiopaque markers are radiodense materials which can be used to outline internal organs and body fluids in CT-scans or X-ray images. Unlike the previous marking techniques, it is possible to follow these markers through the digestive system real time. Consequently, the gastrointestinal motility and the behaviour of digesta in the digestive tract can be analysed for different anatomical compartments. Barium sulphate suspensions are frequently used in passage studies7,29,37,45. However, it is semiquantitative and evaluates the gastric emptying time of the liquid but not the solid fraction of the ingesta. Unless the food is ground to fine particles, barium sulphate will separate in a liquid phase and move at a different rate from the solid fraction. The gastric emptying time is known to be the major variable in affecting the orocolonic transit time in cats46. Radiographical and sectional studies in reptilians have shown that in omnivorous and carnivorous species the stomach is where the holdup is longest47. To intercept this problem Miller et al. (2012)7 defined the intestinal transit time from the initiation of gastric evacuation till the last movement of digesta from the large intestine into the rectum. However, just as in caiman (Caiman crocodylus)37, unexpectedly fast passage times were measured in rainbow skinks (Lampropholis delicate). Despite a non significant difference between their control and barium sulphate studies, the results of the beads control study were overall slower than the barium results. Similar results were reported by Beaupre et al. (1993)23 and Hatch and Afik (1999)5. This might imply that the barium sulphate moves, not only with gastric evacuation, but also in the intestines at the same rate as the fluid-phase of the digesta. Schumacher and Toal (2001)48 discussed the fact that barium sulphate might slow intestinal passage times. Recently a study of Miller et al. (2012)7 did not found such an effect. This could be due to the fact they used only a 0,15 ml dosage instead of the frequently used 10-15 ml/kg dosage7,49. Scintigraphy is a quantitative and non-invasive method of determining the liquid-phase and solidphase of the digesta, but it requires expensive equipment and the use of radio-labelled markers46. Alternatively, Valente et al. (2008)29 used barium-impregnated polyethylene spheres (BIPS®) to measure the passage rate in loggerhead sea turtles (Caretta caretta). The BIPS® are inert, white and have a density similar to food, but are sufficiently radiodense to show up clearly on abdominal radiographs. However, the specific gravity of the spheres turned out to be higher than of the turtle food, and therefore might travel with the solid phase of the digesta. They also described some practical limitations using BIPS® in turtles. Hailey et al. (1998)50 reported difficulties counting the microbeads because a large number of beads was used to outline the gut, and so some beads must have overlapped in X-ray photographs. In Caiman crocodylus and the Crocodylus Porosus the spheroids were separated in the stomach from the digestible food and most stayed in there till 24 days after feeding, long after the digestible food and barium meal had been eliminated36,37. An overall major disadvantage using radiopaque markers is the intensive animal handling because of the frequent radiographic imaging, resulting in higher stress levels of the animals and affected transit times29,51. Digestive Markers Internal markers External markers Pigments and dyes Particulate markers Chemical markers Quanifiable Nonquantifiable Figure 1: Schematic overview of the type of markers used in reptile digestion studies Radiopaque markers Marker selection According to the overview carmine red, chromic oxide, barium sulphate, barium polystyrene beads, and lead glass beads have been used to determine the passage rate in Crocodiliae. The use of these markers, however, resulted in a wide range of transit times. Besides most of these techniques are not easy to recover, especially in non-laboratory conditions, or include intensive animal handling. Since in many reptile studies fluorescent powder, pieces of tape, and beads have successfully been used for determination of transit times, they are incorporated in the present study design. These markers are selected because they are easy to detect, quantitative, inexpensive, do not require extensive lab procedures and are therefore easy to use. A combination of plastic beads, fluorescent powder and pieces of flagging tape is used as digestive markers for determining the minimum and maximum transit times. By using a combination of these markers, which are all different in size and texture, we attempt to get a better understanding of the difference in behaviour of these markers in the digestive tract of C. Porosus. Beads are used in the experimental designs, despite the fact that Davenport et al. (1990)36 and Davenport et al. (1992)37 reported inconvenient results after using beads. However, by repeating the experiments with beads we will be able to compare our results with those reported in the literature. In these articles the main reason for the retained markers in the stomach is thought to be caused by the anatomical and physiological functions of the crocodile stomach. However, in the following experiments juvenile crocodiles are used with a larger body mass than used in previous studies and for the first time sub-adult (culling size) crocodiles are used in a digestion study. From another unpublished study, concerning sub-adult crocodiles (N = 11), it is known that the body size (snoutvent-length) is positively related to the size of the stomach, r = .59, p < 0.05. Therefore it is suspected that the beads will behave differently. We expect that the size of the crocodile, and thus the size of the gastro-intestinal organs, might influence the behaviour of beads within the gastro-intestinal tract. Material and Methods Experiment 1: Digestive passage rate in yearling crocodiles In this experiment 18 yearling crocodiles (mean body weight = 532 g, SD = 97 g; mean SVL = 296 mm, SD = 15 mm) were selected from a captive population (Crocodylus Park, Darwin, Australia). Each individual was randomly assigned to one of three treatment groups (N = 6 per group) that were fed a meal of chopped red meat equivalent to 2% of their body mass1. An inert marker (<1% of meal mass) was mixed with each meal to track the progression of food through the digestive tract. The first group was fed a meal mixed with 15 plastic beads (1mm diameter), the second group was given a meal mixed with 15 pieces (6 mm circles) of plastic field flagging, and the third group was given a meal mixed with 250 mg of finely milled inert plastic pigment (Glow-Mark Fluorescent Pigment; 5-35 μm particle size; Scientific Marking Materials, Seattle, WA). Table 1: Statistical descriptive of Experiment 1 Marker Group N SVL (mm) Sig.* Beads 6 292,5 ± 16,1 504,0 ± 81,8 Flags 6 294,8 ± 14,0 536,8 ± 104,5 Powder 6 300,2 ± 16,4 555,2 ± 113,8 Total 18 295,8 ± 14,9 0,687 BW (g) 532,0 ± 97,3 Sig.* 0,679 * Significance measured between groups The meals were administered via gavage by inserting a lubricated syringe barrel behind the gular valve, which is a structure consisting of the dorsal flap of the tongue and the palatal flap (velum palati) extending from the soft palate52. Food was slowly pushed into the stomach by depressing the syringe plunger. A second and a third meal (2% of body mass), not containing a marker, were given every 3 days to simulate the feeding habits of wild-caught individuals and the consumption of small meals every few days2. The yearling crocodiles were individually housed in plastic tubs (40,0 cm * 30,0 cm) and kept inside a 30 °C constant temperature room. Each tub contained 1.0 L of fresh water, which was changed daily, and included a resting platform to allow crocodiles to climb out of the water or to hide beneath it. Every 24 hours the tubs were checked for the appearance of faeces and the digestive markers for nine days after initial feeding. The number of beads and pieces of flagging that had passed through the digestive tract were counted and a semi-qualitative scale was used to score passage of digesta in the group fed a meal containing finely milled pigment. The pigment reference scale ranged from 0 to 6, corresponding to concentrations of respectively 25 mg pigment diluted into 1 L water, 50 mg L -1, 100 mg L-1, 150 mg L-1, 200 mg L-1, and 250 mg L-1. Scoring of pigment passage was facilitated by using a high power black light as the pigment fluoresced brightly, even at the lowest concentrations. On the ninth day post-feeding, stomach contents were flushed by using a common flushing technique evaluated by Fitzgerald (1989)53, which is near 100% effective in recovering crocodilian stomach contents54. A 10 mm o.d. piece of silicon tubing was inserted behind the gular valve and into the stomach. A constant low velocity flow of tap water was used to fill the stomach cavity until visibly extended. Crocodiles were held in a vertical position while a gentle massaging motion was used to flush the stomach until all stomach contents were collected for enumeration. Experiment 2: Digestive passage rate in culling size crocodiles For this experiment 24 male sub-adult crocodiles (mean SVL = 956 mm, SD = 92 mm) were selected from the same captive population as described above. After being immobilised, by a technique described by Franklin et al. (2003)55, each crocodile was gavage fed an intact chicken head containing 30 beads and 30 pieces of flagging (identical to those used above). These markers were inserted into a size 00 gelatine capsule, which was then placed into the oropharynx of the chicken. To prevent the capsule from falling out of the chicken head, the upper and lower beak were glued together with 2-3 drops of cyanoacrylate glue, and the oesophagus was tied close with a knot. For 12 of these crocodiles, the feeding was accompanied with the insertion of a temperature data logger (TidbiT v2; Onset Comp.) programmed to record core body temperature every 10 minutes. Crocodiles were given a follow up ad libitum meal of 2-3 chicken heads every 2 days following the initial feeding. These crocodiles were housed in individual cages (0.75m * 0.75m * 2,0 m) that were partially immersed into the water of commercial raising ponds. The unitised cages are standard practice for housing farmed crocodiles, and they allow individual feeding, as well as prevent agonistic interactions. The cages also permit free thermoregulation by providing individuals the choice between water and a basking platform. During the experimental period of six days post feeding the inert markers, animals of the corresponding day post feeding group were sacrificed and dissected every 24 hours. The digestive tract was dissected out from the oesophagus to the cloacae, and recorded the location of beads and pieces of flagging along the tract. The digestive tract was separated into five discrete sections; stomach, proximal-intestine, mid-intestine, distal-intestine, and colon. Sections were occluded with cable ties before separation, and the plastic markers were filtered from the digesta using tap water and a series of fine mesh sieves with 0.5-3 mm screen openings. Table 2: Statistical description experiment 2 SVL Days post feeding N Mean Std. Deviation 1 4 957,00 77,02 2 4 928,75 80,04 3 4 952,50 80,39 4 4 977,50 88,13 5 4 940,00 79,97 6 3 986,67 200,08 Total 23 955,78 92,36 Sig.* 0,972 * Significance measured between groups, SVL = Snout Vent Length in mm Statistical analysis For evaluation of the collected data of experiment 1 and 2 statistical analyses were conducted using IBM SPSS statistics 20. De used test for each analysis will be discussed in the following paragraphs. Results Experiment 1 An one-way ANOVA showed no significant differences in snout-vent-length (SVL) (F(2, 15) = 0.394, p > 0.05), or body weight (BW) (F(2, 15) = 0.397, p > 0.05) between the three different marker treatment groups either in experiment 1. Markers were recovered as shown in figure 2. The means and standard deviations of the recovered markers for each day post feeding are presented in table 2. 15 14 13 Average recovered markers 12 11 10 9 8 beads 7 flags 6 5 powder 4 3 2 1 0 Days post feeding Figure 2: Excreted concentrations for each type of marker The collected data of the beads, flags, and powder were checked for normality before running any statistical model. According to the Shapiro-Wilk test of normality not all data were normal distributed. Beads, as well the flags, were non-normal distributed for all days, with exception of the stomach flush. A standardised residual plot was performed showing that all z-scores were within the –1.96 and 1.96 limits. Therefore these data are assumed to be normally distributed data, and an ANOVA model is allowed to use. No flags were recovered during the nine days post feeding. By performing a stomach flush a total of 99% of the flags were recovered. An one-way ANOVA showed that the mean of recovered flags after performing a stomach flush was significantly different (p < 0.01) from the previous days. A single bead was recovered two days post feeding together with some undigested food in the water. Two beads were recovered at day 7, 1 at day 8, and 3 at day 9 post feeding. Each time beads were found between day 1 and day 9 they were found in the same cage, concerning a single crocodile. The results of an one-way ANOVA for repeated measures show that the amount of markers recovered was not significantly affected by the time post feeding, F(8,40) = 0.199, p > 0.05. After performing a stomach flush beads were recovered from all crocodiles, ranging from minimal 1 to a maximum of 14 beads. The means of recovered beads of day 1 till day 8 were all significantly different (p < 0.05) from the stomach flush. Day 9 was not significantly different with a p-value of 0.54. During this experiment 57% of the beads were recovered, where 49% was recovered by performing a stomach flush. Table 3: Average results of recovered markers for each day post feeding Days post feeding Marker Group 1 2 3 4 5 6 Beads 0 0,17 ± 0,41 0 0 0 0 Flags 0 0 0 0 0 0 Pigment 0 1,83 ± 1,67 7 8 9 0,33 ± 0,81 0,17 ± 0,41 0,50 ± 1,22 0 0 0 0,96 ± 1,24 0,50 ± 0,79 0,42 ± 0,56 0,67 ± 0,66 0,17 ± 0,20 0,46 ± 1,01 0,08 ± 0,13 Stomach flush 7,33 ± 5,06a 14,83 ± 0,41b 1,83 ± 0,49c a) Mean of recovered beads after the stomach flush was significantly different from day 1 – 8 post feeding (p < 0,05), b) Mean of recovered flags after the stomach flush was significantly different from day 1 – 9 post feeding (p < 0,01), c) The concentration of pigment is the stomach was significantly different from the concentrations of beads and flags (p < 0,01). Fluorescent powder was first recovered 2 days after feeding within the cages of all six individuals. The concentrations of powder excreted varied greatly among all individual animals during the experimental period. All crocodiles excreted the highest concentrations of powder between day 2 and day 4 followed by another lower peak concentration a few days later. At day 4 post feeding 50% of the initial amount of marker was excreted. The results of the powder-group were mainly non-normal distributed, except for day 2 and the stomach flush. Because of the large variation in data a non-normal distribution is less easy to rule out. Therefore, these data were transformed by calculating the square root of these values. After running a Shapiro-Wilk test of normality with the transformed values, the normality was improved for each day, still day 8 and day 9 remained significant. Also, the Levene’s test for homogeneity of variance changed from significant difference between the variances F(8, 45) = 3.662, p < 0.05 to equal variances for the different days post feeding F(8, 45) = 1.397, p = 0.224. To check whether these values were truly non-normal distributed the standardised residuals were plotted against the standardised predicted values of days post feeding by performing a regression model. The plotted zscores contained a minimal absolute value of -1.013 and a maximum value of 2.622. Since only two standardised residuals (3.33% of the sample cases) have a value greater than 1.96, there is evidence that this model is representative for the actual data set and the data are normally distributed. Based on the assumptions made above, a one-way ANOVA for repeated measures was allowed to evaluate the data. The results of the ANOVA showed that the time post feeding (day 2 – day 9) did not significantly affect the recovered concentration of marker, F(7, 35) = 0.686, p > 0.05. A correlation analysis showed a significant relationship between the recovered concentration of powder and the time post feeding, r = -.472, p < 0.01. The concentration of powder recovered after the stomach flush was not significantly different from the previous days (p > 0.05) To rule out a non-normal distribution of the powder data a non-parametric Friedman’s ANOVA was conducted to evaluate whether there would be major differences in outcome compared with a one- way ANOVA for repeated measures. A post-hoc Wilcoxon signed-rank test was used with a Bonferroni correction to correct for the number of tests. 6 Concentration scale 5 Crocodiles 4 1 3 2 3 2 4 1 5 0 6 Days postfeeding Figure 3: Excretion patterns of the fluorescent pigment for all six individuals The concentration of powder did not significantly change between day 2 and day 9 according to the results of the Friedman’s ANOVA χ2(7) = 13.042 p > 0.05. To compare the results found after the stomach flush with the previous days another test was performed, χ2(8) = 18.332 p < 0.05. A posthoc Wilcoxon test was used to follow up this finding. A Bonferroni correction was applied and so all effects are reported at a 0.5 / 8 = 0.00625 level of significance. Similar to the parametric test it appeared that the concentration of powder did not significantly change between day 2 and the stomach flush, since none of the comparisons had a significance level smaller than 0.00625. To compare the amount of marker that was recovered after the stomach flush between treatments, percentages were used. In order to work with normally distributed data these values were transformed using a Log transformation. An univariate analysis of variance showed that the covariate, type of marker, was significantly related to the percentage of marker that stayed in the stomach, F(1, 14) = 23.86, p < 0.01, r = 0.65. A post-hoc pairwise comparison with an adjusted Bonferroni correction revealed that a treatment with fluorescent powder resulted in significant lower concentration of marker in the stomach compared to treatments with flags (p < 0.01) and beads (p < 0.05). No significant differences were found between treatments with flags and beads (p > 0.05). To evaluate whether a type of marker had an effect on the time of first appearance an univariate analysis of variance was used. Results showed that the time of first appearance was significantly affected by the type of marker that was used, F(1, 14) = 15.0, p < 0.01. A post-hoc pairwise comparison with an adjusted Bonferroni correction revealed that a treatment with fluorescent powder appeared significantly earlier compared to treatments with flags (p < 0.01) and beads (p < 0.01). It was not possible to analyse a relation between the appearance of faeces and the appearance of markers, since in none of the groups faeces could be detected. Experiment 2 An one-way ANOVA was constructed to evaluate whether groups were equally distributed. The test showed no significant differences in SVL between the various days post feeding groups, F(5, 17) = 0.166, p > 0.05. Unfortunately one crocodile was excluded from the experiment because of the appearance of a parasite trail on the skin scutes. About 72,9% of the beads were recovered, and 72,5% were located in the stomach. A single bead was found in the proximal-intestine in a crocodile belonging to the 4 days post feeding group. In one crocodile, from the 3 days post feeding group, two beads were found in the distal-intestine. In three cases beads were found in the oral cavity. Data were checked for normality by performing a Shapiro-Wilk test for normality and Q-Q plots of the standardised residuals. Because data were normally distributed an independent one-way ANOVA was applied to evaluate whether there is an effect of time post feeding on the location of beads in gastro-intestinal tract. Results showed that the time post feeding did not significantly affect the location of beads in the gastro-intestinal tract, F(15, 51) = 0.768, p > 0.05. According to the results of an one-way ANOVA for repeated measures the location of the gastro-intestinal tract had a significant effect on the number of recovered beads, F(1.00, 22.16) = 194.50, p < 0.01. A post hoc test with a Bonferroni correction showed that the numbers of recovered beads in the stomach were significantly different from other intestinal sections for all groups (p < 0.01). Mean recovered beads 30 Days post feeding 25 1 2 3 4 5 6 20 15 10 5 0 Gastro-intestinal sections Figure 4: This figure shows the mean numbers of beads found within the different gastro-intestinal sections for each day post feeding A higher recovery percentage of flags (88,3%) was found, and about 87,1% was recovered within the stomach. Three flags were found in the proximal-intestine; one at 5 days post feeding, two at 6 six days post feeding. Two flags were found 4 days post feeding in the mid-intestine. Three flags were recovered in the distal-intestine at 3,4, and 6 days post feeding. The effect of time post feeding on the gastro-intestinal location was evaluated with an independent one-way ANOVA. Results showed that the time post feeding had no effect on the gastro-intestinal location, F(20, 86) = 1.109 p > 0.05. A one-way ANOVA for repeated measures was used to evaluate the effect of location on the amount of recovered flags. Results showed that the mean numbers of recovered flags in the stomach were significantly different from all other intestinal sections, F(1.03, 22.74) = 714.18, p < 0.01. A pair wise post hoc comparison test with a Bonferroni correction showed that the number of recovered beads in the stomach were significantly different from other intestinal sections for all groups (p < 0.01). Mean recovered flags 30 Days post feeding 25 1 2 3 4 5 6 20 15 10 5 0 Gastro-intestinal sections Figure 5: This figure shows the mean numbers of flags found within the different gastro-intestinal sections for each day post feeding An one-way ANOVA showed no significant differences in mean numbers of recovered markers in the stomach between the several groups post feeding for flags F(5, 17) = 1.557, p > 0,05, and beads F(5, 17) = 0.484, p > 0,05. However a correlation analysis showed that there was a significant relation between the number of days post feeding and the amount of recovered flags in the stomach, r = -.48; p < 0,05. Gastro-intestinal section Stomach Proximal-intestine Mid-intestine Distal-intestine Colon Days post feeding Beads Flags Beads Flags Beads Flags Beads Flags Beads Flags 1 25,00 ± 6,9a 30,00 ± 0b 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 2 20,75 ± 10,1a 27,75 ± 4,5b 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 3 20,00 ± 4,3a 24,00 ± 3,9b 0,00 0,00 0,00 0,00 0,5 ± 1,0 0,25 ± 0,5 0,00 0,00 4 20,25 ± 5,0a 24,00 ± 6,1b 0,25 ± 0,5 0,00 0,00 0,50 ± 1,0 0,00 0,25 ± 0,5 0,00 0,00 25,25 ± 5,1a 24,25 ± 3,8b 0,00 0,25 ± 0,5 0,00 0,00 0,00 0,00 0,00 0,00 b 0,00 0,67 ± 1,2 0,00 0,00 0,00 0,33 ± 0,6 0,00 0,00 0,04 ± 0,2 0,13 ± 0,5 0,00 0,09 ± 0,4 0,09 ± 0,4 0,13 ± 0,3 0,00 0,00 5 a 6 18,33 ± 14,2 Total 21,74 ± 7,4a 23,67 ± 4,0 25,70 ± 4,4b,c Table 4: a) significant difference (p < 0,01) within the same row for beads, b) significant difference (p < 0,01) within the same row for flags, c) significant relation between the number of days post feeding and the number of recovered flags (r = -,48; p < 0,05). A paired sample t-test was used to determine whether there was a difference in the number of markers recovered in the stomach between the two type of markers. Results showed that more flags were recovered in the stomach (M = 25.70, SE = 4.42) than beads (M = 21.74, SE = 7.44), t(22) = 2.42, p < 0.05, r = .46. Results of paired sample t-tests showed that there were no significant differences between the numbers of recovered beads and flags in the stomach for the several days post feeding groups (p > 0.05). Beside markers, various stomach contents as stones, glass and pieces of plastic were recovered within the stomachs of all crocodiles during the dissections. One animal even contained 66 foreign objects. The weight of the recovered corpora aliena varied between 0.5 and 66 grams, with an average of 17 grams. The temperature data loggers showed body temperatures ranging between 23˚C and 30˚C. Figure 6: Body temperatures (mean ± SD) of large sub-adult crocodiles, measured by data loggers in the stomach on a typical day during experiment 2. Discussion The aim of the present research was to determine whether beads, pieces of plastic flagging, and fluorescent pigment can be used as digestive markers in C. porosus. The behaviour of these markers was studied in two experimental designs. In the present study each type of marker clearly behaved differently within the gastro-intestinal tract of the crocodiles. Results showed differences in excretion patterns. The most obvious outcome is that the fluorescent pigment was excreted almost continuously during the experiment, while beads and flags were only excreted sporadically and large percentages were retained within the stomach. In the following paragraphs the results will be discussed for each type of marker and compared with results of the existing literature. Particulate markers Since the numbers of recovered beads did not significantly differ between day 1 and day 9 it can be concluded that the time range of nine days post feeding had no effect on the excretion of beads. The statistical results showed that the number of beads recovered after the stomach flush was significantly different from the previous days, with the exception of day 9, and therefore suggest that about 49% remained in the stomach. Despite the fact that nearly half of the beads stayed in the stomach, some beads passed the digestive tract. Although, the single bead found on the second day post feeding was probably recovered because one animal regurgitated a part of its meal, since little undigested food was found in the water as well. The beads excreted from day 7 till day 9 post feeding only included two individuals. Nevertheless, after performing a stomach flush 57% of the beads were recovered, presuming that 43% have passed the stomach. The number of recovered flags after the stomach flush was significantly different from day 1 to day 9 including. A convincing 99% of the flags were recovered after performing a stomach flush, assuming that just a single flag passed the pylorus into the small intestine. The results of the first experiment were more or less established by the findings of the second experiment, since the time post feeding did not affect the location of beads or pieces of flagging tape in the gastro-intestinal tract, and again, nearly all markers were recovered within the stomach. Only a few markers were found in the intestinal tract. The recovery percentages of the second experiment were prominent. During this trial only 72,9% of the beads and 88,3% of the flagging tape were recovered. The fact that not all markers were recovered assumes that the remaining percentages of markers were not in the digestive tract. This could be caused either because markers moved through the digestive tract before culling, meals were partially regurgitated or markers fell out the animal during bleeding and skinning. During the latter procedures animals are hung up, head down, by their tail. In three animals a couple of beads were found in the oral cavity. Because the animals were housed in individual cages, which were connected to an open pond, markers could have easily disappeared, especially because the beads were transparent and uncoloured. Based on the recovery percentages, the pieces of flagging were more prone to stay in the stomach and were easy to recover. However, a trend was noticeable showing that less pieces were recovered the more time had passed after feeding. Combined with the fact that pieces of tape were found in other parts of the intestine suggests that some movement through the intestinal tract took place. These findings, that large amounts of particulate markers retained in the stomach, resemble with the results reported in previous crocodile digestion studies. But what causes these markers to function in most reptile species and not in crocodilians? The main reason of particulate markers having difficulties with passing the stomach is thought to be caused by the anatomy and physiologic functions of the stomach. Most reptilians have a simple tubular stomach, however it is larger and more outpocketed in crocodilians56. The cardiac sphincter of the stomach occurs at the junction of the oesophagus, with the cardiac sac at the left anterior corner of the stomach. The pyloric region is divided into left and right halves by a thick collar muscle and spongy tissue, which may operate as a gizzard. The pyloric region is much smaller and opens into the duodenum via a powerful pyloric sphincter, which is placed next to the cardiac sphincter on the cranial site of the stomach, forming a large pouch57. Because of the pouch-form stomach, particulate dense material might sink to the bottom away from the sphincter. Also, in crocodilians the pyloric sphincter sorts the stomach contents to make sure only fluid and fine material passes into the small intestine37. In the literature it is discussed to what extent this sorting function plays a role in the collection of particulate materials within the stomach of crocodilians. The presence of gastroliths, a hard object of no caloric value (e.g., a stone, natural or pathological concretion) which is retained in the digestive tract of an animal, is a common finding in crocodile digestion studies. In the present study all stomachs of sub-adult crocodiles contained gastroliths with an average weight of 17 grams. The possible function of gastroliths are reviewed by Taylor et al. (1996)49 and Wings (2007)58. However their actual function in crocodiles remains uncertain. They might contribute to buoyancy control, food processing or both49. Apparently the pyloric sphincter is able to filter small particles like the used markers from the other stomach contents. A study of Davenport et al. (1990)36 reported a similar stasis of particulate markers in the stomach. In this study none of the particulate markers (1 mm) did pass the stomach for up to 24 days in juvenile crocodiles (C. porosus) with an average weight of 154 grams. Another study of Davenport et al. (1992)37, using juvenile Caiman crocodiles of 240-600 grams, a few particulate markers reached the rectum after 52 hours and about 25% of the particulate markers reached the colon after 74 hours. In the latter study two types of markers were used; 1 mm barium polystyrene spheroids and 0,4 mm lead glass beads. Both markers were found in the colon, assuming that the pyloric sorting mechanism couldn’t 59 differentiate between the spheroids and the beads. Figure 7: Stomach of the crocodile According to the results of the present study the pyloric sphincter of the juvenile C. porosus was able to differentiate between the fluorescent pigment and the particulate markers, and therefore the differences found in excretion patterns are primarily caused by the ability of the marker to pass the pyloric sphincter. So, it can be concluded that a very small particle size is needed to pass the pyloric sphincter. In the present study was hypothesised that the size of the crocodile, and thus the size of the gastrointestinal organs, would have an effect on the filter function of the pyloric sphincter. It was expected that pyloric sphincter in larger crocodiles would let through relatively larger particles. However, according to the results of the present study there were no differences in marker passage between the two experiments, neither compared with previous reported studies in which smaller animals were used. Apparently animal size has no influence on the filter function of the pyloric sphincter and even sub-adult animals are still able to select particles < 1 mm. The beads in the present study behaved similarly to the way Davenport et al. (1992)37 described the movement of beads in Caiman crocodiles, except for the fact that a few particles could already be detected in the rectum after 54 hours. Even though the appearance of the markers in the faeces was not recorded, it seems that markers travelled at a faster rate compared with the present results. They reported that the pyloric sphincter was unable to differentiate between 0.4 or 1 mm beads, so the size of the beads used in the present study shouldn’t have caused the delay in transit time of the marker. However, the feeding frequency could play a part in this, since Davenport et al. (1992)37 noticed that each time a new (non-labelled) meal was eaten the particulate markers were again dispersed throughout the stomach, and a few particles left the stomach. In their study crocodiles were fed unlabelled food every day. In the present study the crocodiles were fed an unlabelled meal every three days at day 3 and day 6. This could explain the fact that beads were excreted after 7 days post feeding, just like the fact that 43% of the beads passed the stomach. In both studies two unlabelled meals were necessary prior to the passage of beads through the pyloric sphincter. Another factor that might have had an impact on the passage rate of the markers is the frequency of animal handling. In the present study animals were gavage fed, because a previous trial with voluntarily feeding was not successful. Crocodiles didn’t immediately take in their experimental diet which made it hard to control whether they had consumed the whole diet including all markers. Besides the gavage feeding animals were handled every day when their tubs were cleaned during the first experiment. In order to gavage feed the crocodiles in the second experiment the crocodiles needed to be immobilised. A comparative study of Franklin et al. (2003)55 showed that crocodiles (C. porosus) returned at their physiological baseline levels approximately 8 hours after manual restraint. The stress response was significantly reduced compared to stunned animals. Guilliette et al. (1997)60 reported an approximate 30-fold increase in plasma corticosterone levels in juvenile alligators subjected to two hours of capture and restraint. It is known from avian literature that corticosterone slow down the passage rate of food in the alimentary tract51. It is uncertain to what extent the way and frequency of animal handling in the present study had impact on the passage rate, but it might have contributed to the current results. Also, the environmental temperature has a major influence on the digestive system of ectotherm species. Digestion in crocodiles is directly affected by temperature; increasing temperatures result in increased appetite, gastric contraction frequency and amplitude, and peptic activity61. Efficient body temperatures for ingestion and digestion for crocodiles are estimated between 25˚C and 35˚C. Temperatures higher than 35˚C cause an undue amount of stress and inappetence62, however Coulson et al. (1981)63 reported ceased feeding in Alligator mississippiensis at temperatures below 22˚C. Caiman crocodiles even regurgitate their food when thermal regimes are unsuitable for digestion64. According to the data loggers used in experiment 2 body temperatures ranged between 23˚C and 30˚C. During the day time temperatures were high enough to support digestion. Also, since no major differences were found compared to experiment 1, wherein the environmental temperature was controlled at a constant 30˚C, it is unlikely that the environmental temperature affected the excretion rate of beads. Therefore, regurgitation is unexpected to be the main reason for the missing markers in experiment 2. In the study of Davenport et al. (1992)37 only two crocodiles were used to compare the effect of particulate markers. The way these markers behaved were extremely different for both individuals. One of them excreted all markers after 168 hours, while the other animal had still substantial numbers of markers within the stomach after 192 hours. This is in agreement with the findings of experiment 1, wherein two animals still contained 14 beads in the stomach after nine days, one animal 10 beads, and the other crocodiles 3, 2, and 1 beads. So apparently not all juvenile crocodiles selected stomach contents to the same extend, so individual variation is a major opponent when using this marker, even under controlled conditions. The pieces of plastic flagging tape obviously did not work as digestive markers in this study. Despite the fact that the material is pliable and light, it is also prone to adhesion to the intestinal wall8, or in this case the mucosa of the stomach. Especially under wet circumstances the tape becomes sticky, so it is also very likely that the several pieces of tape clumped together within the stomach, making it even more difficult to pass the pyloric sphincter. When looking at the overview of digestive markers, it is remarkable that this type of marker has been mostly successful in herbivorous or opportunistic omnivorous reptiles. The reason for the fact that mainly herbivores are able to excrete this type of marker might be explained by their diet. Among herbivorous species some have teeth (sauria) and others don’t (chelonie)65. In both cases animals are anatomically designed to tear leaves and preclude mastication of food66. The lack of particle size reduction results in digestion of large food particles, such as entire leaves13. In most species the stomach is not capable to fully break down these particles. Therefore relatively large particles pass the pylorus into the small intestine to be further fermented in the hindgut. In contrast to the crocodile stomach it is more likely for markers to pass through. Fluorescent pigment In contrast to the particulate markers the fluorescent pigment was the only marker that passed the stomach in large amounts. It was first detected two days post feeding within the tubs of all six individuals, and 50% of the marker was recovered at day 4. Although the concentrations of powder excreted varied greatly among the individual animals a trend in excretion was noticed. According to the statistical results, the time post feeding seemed not to affect the concentration of excreted pigment, since there were no significant differences in excretion between day 2 and day 9. However, the time post feeding was significant negatively related to the excreted concentration of pigment. This trend is also visible in figure 3. Besides, for almost all crocodiles their maximum concentration of excreted marker was detected on day 2 or day 3, followed by another peak concentration a few days later. In comparison with the other markers, the concentration of pigment recovered after the stomach flush did not significantly differ from the previous days. Nevertheless, the fact that some pigment was retained by the stomach flush indicates that not all pigment had left the stomach after nine days. Yet, what do these results mean? The fluorescent pigment is thought to travel at the same rate as the solid fraction of the diet. In a study of Beaupre et al. (1993)23 the liquid phase appeared long before the with pigment stained faeces. However, in a study of Hatch and Afik. (1999)5 it travelled at almost the same rate as the liquid fraction in Six-lined racerunners (Cnemidophorus sexlineatus). They concluded that the ability to separate the liquid fraction from the solid fraction differs among species. In the literature it is unknown to what extent crocodile species are able to separate these fractions of the diet. It is known that barium sulphate is evacuated from the stomach at the same rate as the liquid phase. Davenport et al. (1992)37 noticed that barium sulphate labelled material first left the stomach after 4 hours post feeding and was detected in the rectum in as little as 12 hours in Caiman crocodiles, which probably represented the liquid fraction. After 52 hours all barium sulphate labelled material filled the rectum. A first appearance of faeces stained with chromic oxide, which is known not to travel with any fraction of the diet, was reported around 49 hours post feeding and a mean last appearance after 136 hours. Davenport et al. (1990)36 reported a first appearance of faeces stained with chromic oxide after 68 hours and mean last appearance after 97 hours in C. porosus. Garnett (1988)1 recorded the first appearance of carmine red after 36 hours and the last appearance at 96 hours in C. porosus using a diet of lean pork. He reported a first appearance after 24 hours and a last appearance after 48 hours when using a fatty pork diet. The fact that the pigment was first detected after 48 hours seems plausible compared to other data. Apparently it took at least 24 hours for the pigment to pass the digestive tract to be expelled. Unfortunately the excretion of the pigment could not be related to the defecation pattern, since the faeces could not be collected. The faeces was easily diffused within the water which made it impossible to detect and the clarity of the water was affected by the repellence of skin. However, when looking at the excretion patterns of the pigment it seems likely that most of the experimental diet was excreted within the first few days. After that, only small concentrations were excreted, which are probably caused by internal staining of the following meals, as described by Harwood (1979)8. Therefore the pigment might be a usable marker to determine the first appearance, but not to estimate the maximal transit time. However, because of the large time intervals in this study design, the pigment might be excreted between 24 and 48 hours. Additionally, the fact that the pigment concentrations were scaled and estimated by visual detection makes the data even less accurate. Consequently, the present data can only be used as rough estimations of the passage rate. Future research Nevertheless, up till now most results reported in crocodile digestion studies have been rough estimations of food passage rates. Besides, all study designs differ in ways of the used species, experimental diet, feeding frequency, time interval, housing, recovering the marker, etc. This way there are too many biases involved to compare the results from the previous studies and get a clear view of the rate at which food is processed in crocodiles. However, with the present collection of data and knowledge a basis of information about the food passage rate in crocodiles is formed. It can be concluded that crocodiles are not the easiest and well willing species to work with, and not just because they bite! Because of the anatomical and physiological restraints of the crocodile alimentary tract more advanced digestive markers are needed compared to other reptiles in order to obtain accurate data. Since the size of the marker seems to be the main limitation for a marker to be successful in crocodilians, the ideal marker needs to be small enough to pass the pyloric sphincter. To make sure it moves at the same rate along the intestinal tract as the diet ideally it should be attached to a certain feedstuff. Therefore a chemical marker might be a solution. Although the detection will require special equipment it has some major advantages like the fact that they are quantifiable markers, and by using several chemical markers for the different fractions of the digesta, it will be possible to provide knowledge about the ability of crocodiles to separate the solid phase from the liquid phase. With these markers it is possible to strip an experimental diet and estimate passage rates for several nutrients. Also, animal handling is still minimal involved since only the faeces needs to be collected. In the present study it was not possible to collect the faeces. So attention is needed when designing the experimental environment for future experiments. In terms of animal handling, in the present study animals were gavage fed, however to reduce the influence of stress on the passage time, future research should aim for voluntary feeding designs. Additionally the animal handling should be minimised during the experiment. Other implications for future research concern the individual variation in digestion among crocodiles. Previous studies worked with very small population sizes. In order to intercept the large impact of individual variation on test results, experimental populations should be large enough and the environment should be controlled. Also by standardization of certain procedures within future experimental designs will enhance the ability to compare data and indirectly enlarge the population. By increasing the frequency of control during the experiment, not only data will become more accurate, it will also reduce the differences between individuals Conclusion The used particulate markers in this experiment, beads and pieces of plastic flagging, are not appropriate digestive markers for C. porosus. Large percentages of both markers were retained within the stomach. The anatomy and physiological functions of the stomach are thought to have a major role in this occurrence. Because of the small particle size of the fluorescent pigment, it was able to pass the pyloric sphincter and was excreted almost continuously. However, because it is unknown which fraction of the diet was measured and the inaccurate way of recovering the marker, the results can only be interpreted as rough estimations of food transit time. For years crocodile digestion studies are based on rough estimations of passage rates. In order to provide more accurate data concerning the passage rate of food, a more precise markers needs to be used in crocodilians. Over the last few years more accurate digestive markers have been developed and more knowledge about their behaviour and effectiveness is available in various animal species. By using chemical markers as digestive markers other doors in crocodile digestion and nutrition research will be opened. Literature 1. Garnett S. Digestion, assimilation and metabolism of captive estuarine crocodiles, crocodylus porosus. Comparative Biochemistry and Physiology Part A: Physiology. 1988;90(1):23-29. doi: 10.1016/0300-9629(88)91000-6. 2. Webb G, Manolis SC, Whitehead PJ. Wildlife management: Crocodiles and alligators. Chipping: S. Beatty & Sons. Norton, NSW; 1987. 3. Staton MA, Edwards Jr HM, Brisbin Jr IL, Joanen T, McNease L. The influence of environmental temperature and dietary factors on utilization of dietary energy and protein in purified diets by alligators, alligator mississippiensis (Daudin). Aquaculture. 1992;107(4):369-381. 4. Zimmerman LC, Tracy CR. Interactions between the environment and ectothermy and herbivory in reptiles. Physiol Zool. 1989;62(2):374-409. doi: 10.2307/30156176. 5. Hatch KA, Afik D. Retention time of digesta in insectivorous lizards—a comparison of methods and species. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 1999;124(1):89-92. doi: 10.1016/S1095-6433(99)00094-X. 6. Christian KA, Tracy CR, Porter WP. Diet, digestion, and food preferences of Galapagos land iguanas. Herpetologica. 1984;40(2):205-212. http://www.jstor.org/stable/3892078. 7. Miller AK, Erasmus BFN, Alexander GJ. Gut and intestinal passage time in the rainbow skink (Trachylepis margaritifer): Implications for stress measures using faecal analysis. J Anim Physiol Anim Nutr. 2012:no-no. doi: 10.1111/jpn.12004. 8. Harwood RH. The effect of temperature on the digestive efficiency of three species of lizards, Cnemidophorus tigris, Gerrhonotus multicarinatus and Sceloporus occidentalis. Comparative Biochemistry and Physiology Part A: Physiology. 1979;63(3):417-433. doi: 10.1016/03009629(79)90613-3. 9. Waldschmidt SR, Jones SM, Porter WP. The effect of body temperature and feeding regime on activity, passage time, and digestive coefficient in the lizard Uta stansburiana. Physiol Zool. 1986;59(3):376-383. http://www.jstor.org/stable/30156109. 10. Barboza PS. Digesta passage and functional anatomy of the digestive tract in the desert tortoise (Xerobates agassizii). Journal of Comparative Physiology B. 1995;165(3):193-202. doi: 10.1007/BF00260810. 11. McConnachie S, Alexander GJ. The effect of temperature on digestive and assimilation efficiency, gut passage time and appetite in an ambush foraging lizard, Cordylus melanotus melanotus. Journal of Comparative Physiology B. 2004;174(2):99-105. doi: 10.1007/s00360-003-0393-1. 12. Iverson JB. Colic modifications in iguanine lizards. J Morphol. 1980;163(1):79-93. doi: 10.1002/jmor.1051630110. 13. Iverson JB. Adaptations to herbivory in iguanine lizards. In: G.M. Burghardt ASR, ed. Iguanas of the world. Park Ridge, N.J.: Noyes Publications; 1982:60-76. 14. Greenwald OE, Kanter ME. The effects of temperature and behavioral thermoregulation on digestive efficiency and rate in corn snakes (Elaphe guttata guttata). Physiol Zool. 1979;52(3):398408. http://www.jstor.org/stable/30155760. 15. Sadeghayobi E, Blake S, Wikelski M, Gibbs J, Mackie R, Cabrera F. Digesta retention time in the galápagos tortoise (Chelonoidis nigra). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2011;160(4):493-497. doi: 10.1016/j.cbpa.2011.08.008. 16. Meance S, Cayuela C, Turchet P, Raimondi A, Lucas C, Antoine J. A fermented milk with a bifidobacterium probiotic strain DN-173 010 shortened oro-fecal gut transit time in elderly. Microbial Ecology in Health & Disease. 2001;13(4):217-222. doi: 10.1080/089106001753341291. 17. Sands JS, Ragland D, Baxter C, Joern BC, Sauber TE, Adeola O. Phosphorus bioavailability, growth performance, and nutrient balance in pigs fed high available phosphorus corn and phytase. Journal of Animal Science. 2001;79(8):2134-2142. 18. Fris MB, Horn MH. Effects of diets of different protein content on food consumption, gut retention, protein conversion, and growth of Cebidichthys violaceus (Girard), an herbivorous fish of temperate zone marine waters. J Exp Mar Biol Ecol. 1993;166(2):185-202. doi: 10.1016/00220981(93)90218-D. 19. Horn MH, Mailhiot KF, Fris MB, McClanahan LL. Growth, consumption, assimilation and excretion in the marine herbivorous fish Cebidichthys violaceus (Girard) fed natural and high protein diets. J Exp Mar Biol Ecol. 1995;190(1):97-108. doi: 10.1016/0022-0981(95)00034-O. 20. Liesegang A, Hatt J, Nijboer J, Forrer R, Wanner M, Isenbügel E. Influence of different dietary calcium levels on the digestibility of ca, mg, and P in captive-born juvenile Galapagos giant tortoises (Geochelone nigra). Zoo Biol. 2001;20(5):367-374. doi: 10.1002/zoo.1035. 21. Kanui T, Mwendia C, Aulie A, Wanyoike M. Effects of temperature on growth, food uptake and retention time of juvenile nile crocodiles (Crocodylus niloticus). Comparative Biochemistry and Physiology Part A: Physiology. 1991;99(3):453-456. doi: 10.1016/0300-9629(91)90032-8. 22. Bjorndal K. Flexibility of digestive responses in two generalist herbivores, the tortoises Geochelone carbonaria and Geochelone denticulata. Oecologia. 1989;78(3):317-321. doi: 10.1007/BF00379104. 23. Beaupre SJ, Dunham AE, Overall KL. The effects of consumption rate and temperature on apparent digestibility coefficient, urate production, metabolizable energy coefficient and passage time in canyon lizards (Sceloporus merriami) from two populations. Funct Ecol. 1993;7(3):273-280. http://www.jstor.org/stable/2390205. 24. Fuller G, Margulis SW, Santymire R. The effectiveness of indigestible markers for identifying individual animal feces and their prevalence of use in North American zoos. Zoo Biol. 2011;30(4):379398. doi: 10.1002/zoo.20339. 25. Read N, Miles C, Fisher D, et al. Transit of a meal through the stomach, small intestine, and colon in normal subjects and its role in the pathogenesis of diarrhea. Gastroenterology. 1980;79(6):12761282. http://europepmc.org/abstract/MED/7439633. 26. Kummrow MS, Gilman C, Mackie P, Smith DA, Mastromonaco GF. Noninvasive analysis of fecal reproductive hormone metabolites in female veiled chameleons (Chamaeleo calyptratus) by enzyme immunoassay. Zoo Biol. 2011;30(1):95-115. doi: 10.1002/zoo.20318. 27. Troyer K. Diet selection and digestion in Iguana iguana: The importance of age and nutrient requirements. Oecologia. 1984(2): 201. doi: 10.1007/BF00396761. 28. Wikelski M, Gall B, Trillmich F. Ontogenetic changes in food intake and digestion rate of the herbivorous marine iguana (Amblyrhynchus cristatus, bell). Oecologia. 1993;94(3):373-379. http://dx.doi.org/10.1007/BF00317112. doi: 10.1007/BF00317112. 29. Valente A, Marco I, Parga M, Lavin S, Alegre F, Cuenca R. Ingesta passage and gastric emptying times in loggerhead sea turtles (Caretta caretta). Res Vet Sci. 2008;84(1):132-139. doi: 10.1016/j.rvsc.2007.03.013 30. Hailey A. The specific dynamic action of the omnivorous tortoise Kinixys spekii in relation to diet, feeding pattern, and gut passage. Physiol Zool. 1998;71(1):57-66. http://www.jstor.org/stable/10.1086/515883. 31. Spencer R, Thompson MB, D. Hume I. The diet and digestive energetics of an australian shortnecked turtle, Emydura macquarii. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 1998;121(4):341-349. doi: 10.1016/S1095-6433(98)10132-0. 32. Bjorndal KA. Diet mixing: Nonadditive interactions of diet items in an omnivorous freshwater turtle. Ecology. 1991:1234-1241. 33. Brand SJ, Lanyon JM, Limpus CJ. Digesta composition and retention times in wild immature green turtles, Chelonia mydas: A preliminary investigation. Mar Freshwater Res. 1999;50(2):145-147. http://www.publish.csiro.au/paper/MF98033. 34. Amorocho DF, Reina RD. Intake passage time, digesta composition and digestibility in east pacific green turtles (Chelonia mydas agassizii) at gorgona national park, colombian pacific. J Exp Mar Biol Ecol. 2008;360(2):117-124. doi: 10.1016/j.jembe.2008.04.009. 35. Titgemeyer EC. Design and interpretation of nutrient digestion studies. J Anim Sci. 1997;75(8):2235-2247. http://www.journalofanimalscience.org.proxy.library.uu.nl/content/75/8/2235.abstract 36. Davenport J, Grove D, Cannon J, Ellis T, Stables R. Food capture, appetite, digestion rate and efficiency in hatchling and juvenile Crocodylus porosus. J Zool. 1990;220(4):569-592. 37. Davenport J, Andrews T, Hudson G. Assimilation of energy, protein and fatty acids by the spectacled caiman Caiman crodocilus crocodilus L. Herpetological journal. 1992;2(3):72-76. 38. Austreng E, Storebakken T, Thomassen MS, Refstie S, Thomassen Y. Evaluation of selected trivalent metal oxides as inert markers used to estimate apparent digestibility in salmonids. Aquaculture. 2000;188(1):65-78. doi: 10.1016/S0044-8486(00)00336-7 39. Bernard L, Doreau M. Use of rare earth elements as external markers for mean retention time measurements in ruminants. Reproduction Nutrition Development. 2000;40(2):89-102. doi: 10.1051/rnd:2000122 40. Davies S, Gouveia A. Comparison of yttrium and chromic oxides as inert dietary markers for the estimation of apparent digestibility coefficients in mirror carp (Cyprinus carpio) fed on diets containing soybean‐, maize‐and fish‐derived proteins. Aquacult Nutr. 2006;12(6):451-458. doi: 10.1111/j.1365-2095.2006.00448.x 41. Krockenberger M, Bryden M. Rate of passage of digesta through the alimentary tract of southern elephant seals (Mirounga leonina)(carnivora: Phocidae). J Zool. 1994;234(2):229-237. doi: 10.1111/j.1469-7998.1994.tb06071.x 42. Sundling L, Ahlstrøm Ø, Tauson A. Comparative digestibility of nutrients and energy in ferrets (Mustela putorius furo), mink (Neovison vison) and cats (Felis catus). Proceedings of the Xth International Scientific Congress in fur animal production . Wageningen Academic Publishers. 2012:112-120. doi: 10.3920/978-90-8686-760-8_12 43. Vhile S, Skrede A, Ahlstrøm Ø, Hove K. Yttrium oxide (Y2O3) as an inert marker in digestibility studies with dogs, blue foxes and mink fed diets containing different protein sources. J Anim Physiol Anim Nutr. 2007;91(9‐10):381-389. doi: 10.1111/j.1439-0396.2006.00665.x 44. Tracy CR, Zimmerman LC, Tracy C, Bradley KD, Castle K. Rates of food passage in the digestive tract of young desert tortoises: Effects of body size and diet quality. Chelonian Conservation and Biology. 2006;5(2):269-273. doi: 10.2744/1071-8443(2006)5[269:ROFPIT]2.0.CO;2. 45. Herrel A, Verstappen M, De Vree F. Modulatory complexity of the feeding repertoire in scincid lizards. Journal of Comparative Physiology A. 1999;184(5):501-518. doi: 10.1007/s003590050350 46. Chandler ML, Guilford G, Lawoko CR. Radiopaque markers to evaluate gastric emptying and small intestinal transit time in healthy cats. Journal of Veterinary Internal Medicine. 1997;11(6):361-364. doi: 10.1111/j.1939-1676.1997.tb00481.x 47. Guard CL. The reptilian digestive system: General characteristics. In: Schmidt-Nielsen K, Bolis L, Taylor CR, Bentley PJ, Stevens CE, ed. Comparative physiology: primitive mammals. Cambridge Univ Press; 1980:43-51. 48. Schumacher J, Toal RL. Advanced radiography and ultrasonography in reptiles. Seminars in Avian and Exotic Pet Medicine. 2001;10(4):162-168. doi: 10.1053/saep.2001.24671 49. Taylor SK, Citino SB, Zdziarski JM, Bush RM. Radiographic anatomy and barium sulfate transit time of the gastrointestinal tract of the leopard tortoise (Testudo pardalis). Journal of Zoo and Wildlife Medicine. 1996;27(2):180-186. http://www.jstor.org.proxy.library.uu.nl/stable/20095563 50. Hailey A, Chidavaenzi RL, Loveridge JP. Diet mixing in the omnivorous tortoise Kinixys spekii. Funct Ecol. 1998;12(3):373-385. doi: 10.1046/j.1365-2435.1998.00203.x. 51. Nasir A, Moudgal R, Singh N. Involvement of corticosterone in food intake, food passage time and in vivo uptake of nutrients in the chicken (Gallus domesticus). Br Poult Sci. 1999;40(4):517-522. doi: 10.1080/00071669987296 52. Putterill JF. A morphological study of the oral cavity, pharyngeal cavity and oesophagus of the Nile crocodile, Crocodylus niloticus (Laurenti, 1768). Dissertation, Master of Science, University of Pretoria. 2002. http://upetd.up.ac.za/thesis/submitted/etd-08132008- 085752/unrestricted/dissertation.pdf 53. Fitzgerald LA. An evaluation of stomach flushing techniques for crocodilians. J Herpetol. 1989;23(2):170-172. http://www.jstor.org.proxy.library.uu.nl/stable/1564024 54. Rice A, Ross J, Finger A, Owen R. Application and evaluation of a stomach flushing technique for alligators. Herpetological Review. 2005;36(4):400-401. 55. Franklin CE, Davis BM, Peucker S, et al. Comparison of stress induced by manual restraint and immobilisation in the estuarine crocodile, Crocodylus porosus. Journal of Experimental Zoology Part A: Comparative Experimental Biology. 2003;298(2):86-92. doi: 10.1002/jez.a.10233 56. Stevens CE, Hume ID. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol Rev. 1998;78(2):393-427. 57. Grigg G, Gans C. Morphology and physiology of the crocodylia. In: Fauna of Australia: Amphibia and Reptilia, Australian Government Publishing Service . 1993;40(2a): 326-336 http://espace.library.uq.edu.au/view/UQ:9776. 58. Wings O. A review of gastrolith function with implications for fossil vertebrates and a revised classification. Acta Palaeontol Pol. 2007;52(1):1-16. http://app.pan.pl/archive/published/app52/app52-001 59. Chest of books. Reptilia, part 9. http://chestofbooks.com/animals/zoology/Anatomy/ReptiliaPart-9.html#.U8EmpZR_ua8. 60. Guillette Jr LJ, Crain DA, Rooney AA, Woodward AR. Effect of acute stress on plasma concentrations of sex and stress hormones in juvenile alligators living in control and contaminated lakes. J Herpetol. 1997;31(3):347-353. http://www.jstor.org.proxy.library.uu.nl/stable/1565662 61. Lang JW. Crocodilian thermal selection. In: Wildlife management: crocodiles and alligators. Surrey Beatty & Sons Pty Ltd., Chipping Norton, NSW, Australia. 1987:301-317. 62. Lane T. Crocodilians - digestive system. In: Mader DR, ed. Reptile Medicine and Surgery. Elsevier Inc., Philadelphia; 2006:107-108. 63. Coulson RA, Herbert J. Relationship between metabolic rate and various physiological and biochemical parameters. A comparison of alligator, man and shrew. Comparative Biochemistry and Physiology Part A: Physiology. 1981;69(1):1-13. doi: 10.1016/0300-9629(81)90632-0 64. Diefenbach CO da. Regurgitation is normal in Crocodylia. Ciencia e Cullura (S. Paulo); 1981;33:8283. 65. Bjorndal KA. Fermentation in reptiles and amphibians. In: Mackie RI, White BA. Gastrointestinal microbiology. Springer; 1997:199-230. doi: 10.1007/978-1-4615-4111-0_7 66. Norman DB, Weishampel DB. Ornithopod feeding mechanisms: Their bearing on the evolution of herbivory. Am Nat. 1985;126(2):151-164. http://www.jstor.org.proxy.library.uu.nl/stable/2461504 67. Meienberger C, Wallis IR, Nagy KA. Food intake rate and body mass influence transit time and digestibility in the desert tortoise (Xerobates agassizii). Physiol Zool. 1993;66(5):847-862. http://www.jstor.org/stable/30163827. 68. Damme RV, Bauwens D, Verheyen RF. The thermal dependence of feeding behaviour, food consumption and gut-passage time in the lizard Lacerta vivipara Jacquin. Funct Ecol. 1991;5(4):507517. http://www.jstor.org/stable/2389633. 69. Valido A, Nogales M. Digestive ecology of two omnivorous canarian lizard species (Gallotia, lacertidae). Amphibia-Reptilia. 2003;24(3):331-344. doi:10.1163/156853803322440790". 70. Franz R, Hummel J, Müller DW, Bauert M, Hatt J, Clauss M. Herbivorous reptiles and body mass: Effects on food intake, digesta retention, digestibility and gut capacity, and a comparison with mammals. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2011;158(1):94-101. doi: 10.1016/j.cbpa.2010.09.007 Appendix Table 5: Overview reptile digestion studies Author Species Food habit Marker Garnett (1988)1 Crocodylus Porosus Carnivore Carmine red suspension Liesegang et al. (2001) 20 Kanui et al. (1990)21 Harwood (1979)8 Size of marker Dosage Administration 1,0 ml in a meal (2% of body weight) Behind palatal flap before feeding Geochelone nigra Herbivore Carmine red 66 mg/ Kg body weight Mixed in food Crocodylus niloticus Carnivore Indigo carmine solution Concentration solution unknown 2 g beef soaked in indigo carmine solution Sceloporus occindentalis Carnivore Indigo carmine solution Concentration solution unknown Injected in mealworms Tb ˚C Transit time Def. of passage time Lean pork 30 36-96 hours t0-tmax Fatty pork 30 24-48 hours t0- tmax 23 8-18 days t0- tmax 25 34,8h ± 1,5 t0 25-30 42,3h ± 1,0 t0 30 44,0h ± 0,8 t0 20,8 >9 days t50 26,2 2,8 ± 0,2 days t50 29,2 2,9 ± 0,2 days t50 32,5 2,9 ± 0,2 days t50 33,4 2,4 ± 0,2 days t50 36,6 3,4 ± 0,5 days t50 Experimental diet Beef Tenebrio larvae Gerrhonotus multicarinatus Carnivore Indigo carmine solution Vinyl Cnemidophorus tigris Carnivore Concentration solution unknown < 2mm in diameter Indigo carmine solution Vinyl 1 Concentration solution unknown < 2mm in diameter 1 Injected in mealworms Force fed with mealworms Injected in mealworms Force fed with mealworms Tenebrio larvae Tenebrio larvae Tenebrio larvae Tenebrio larvae 14,9 >11 days t50 18,1 7,6 ± 1,8 days t50 23 5,2 ± 0,3 days t50 27,7 5,9 ± 0,7 days t50 29,9 5 ± 0,3 days t50 32,4 5,1 ± 1,3 days t50 14,9 >11 days t50 18,1 >11 days t50 23 6 ± 0,5 days t50 27,7 7,5 ± 1,0 days t50 29,9 5,2 ± 0,3 days t50 32,4 4,9 ± 1,4 days t50 25,1 3,5 ± 0,4 days t0 28,3 2,9 ± 0,5 days t0 34,3 2,8 ± 0,3 days t0 38,4 2,7 ± 0,7 days t0 25,1 3,6 ± 0,6 days t0 28,3 3,5 ± 0,3 days t0 34,3 2,4 ± 0,4 days t0 38,4 2,9 ± 0,5 days t0 Bjorndal et al. (1989)22 Geochelone carbonaria Geochelone denticulata Beaupre et al. (1993) 23 Hatch and Afik (1999)5 Hailey (1998)30 Sceloporus merriami Cnemidophorus sexlineatus Kinixys spekii Herbivore Herbivore Insectivore Insectivore Omnivore Fluorescenting pigment dye Concentration unknown Fluorescenting pigment dye Concentration unknown UV-powder 0,02 ml per cricket Dyed guava fruit Psidium guajava 22-32 2,6 ± 0,1 days t0 Dyed mango fruit Mangifera indica 22-32 6,6 ± 1,2 days t0 Dyed lantana foliage Lantana urticifolia 22-32 9,5 ± 1,7 days t0 Dyed guava fruit Psidium guajava 22-32 3,6 ± 0,9 days t0 Dyed mango fruit Mangifera indica 22-32 4,8 ± 1,3 days t0 Dyed lantana foliage Lantana urticifolia 22-32 8,7 ± 1,9 days t0 Injected in crickets, free feeding Acheta domestica 31 104,23 h tmax 34 86,54 h tmax 36 113,78 h tmax 28-36 23.3 ± 1,65; 23.3 ± 1,65 h t0; MRT UV-powder 0,02 ml per cricket Injected in crickets, free feeding Polyethylene glycol 0.5 mCi [(U)14C] Gavaged with 30 µl of a solution 28-36 23,7 ± 3,34; 28.8 ± 5.81 h t0; MRT Glycerol triether 2 mCi [3H] Gavaged with 30 µl of a solution 28-36 20,1 ± 2,4; 26,4 ± 6,64 h t0; MRT 50 or 100 loops Sewed into fungi Brassica oleracea 30 2,8; 3,7; 3,3 days t0; t50; tmax Sewed into leaves Brassica oleracea 30 2,2; 4,7; 4,5 days Polyester thread Loops 2,0 cm in diameter Crickets Tied around millipedes Hailey (1998)30 Kinixys spekii Omnivore Brand et al. (1999)33 Xerobates Chelonia mydas agassizii Herbivore Omnivore 30 6,5; 7,2; 7,22 days Radiopaque Ballotini microbeads 0,65-0,75 mm beads 0,5 g (1300) beads Mixed in food, free feeding Polyester thread 2,0 cm in diameter 100 Sewed into leaves Brassica oleracea Field 5 days t50 Sewed into fungi Agaricus bisporus Field 5 days t50 Tied around millipedes Alloporus sp. Field 9 days t50 Mixed with leaves and millipedes 30 3,0; 4,2; 4,2 days t0; t50; tmax Mixed with leaves 30 2,2; 4,7; 4,5 days t0; t50; tmax Chromic oxide Meienberger et al. (1993)67 Alloporus sp. Plastic marker strips Cylindrical polythene beads 0,5 % of wet mass diet 30 * 1-2 mm 2,0-2,5 mm * 1 mm, packed per 40 into gelatine capsules 8 5 capsules 30 No complete results Force fed begin study Erodium cicutarium 16,433,6 17,8 days (1133) t0 Force fed begin study Schismus barbatus 16,433,6 21,8 days (14,5-33) t0 Force-fed Field 15-28 6,5-13,5 days t50 Amorocho and Reina (2008) 34 Van Damme et al. (1991)68 Bjorndal et al. (1991)32 Spencer et al. (1998) 31 Chelonia mydas agassizii Omnivore Cylindrical plastic beads 2,0-3,0 mm *1 mm, packed per 20 into gelatine capsules 3-5 capsules Force-fed Lacerta Vivipara Insectivore Pieces of plastic 3*2*0,1 mm 1 Placed in abdomen cricket, free feeding Trachemys scripta scripta Emydur macquarii Omnivore Omnivore Plastic flagging Vinyl tape Size of duckweed 1x1 mm 1 40 Placed in mouth turtle Put in small sample of fish Leaves and fish 28,3 22 ± 6,3 days; 24,7 ± 6,0 days t0; tmax 20 18,8 ± 1,9 hours t0 25 13,5 ± 1,3 hours t0 27,5 14,0 ± 1,4 hours t0 30 11,2 ± 0,8 hours t0 32,5 10 ± 0,8 hours t0 35 13,5 ± 0,7 hours t0 Duckweed 24-31 72 ± 26 hours t0 Tenebrio larvae 24-31 71 ± 15 hours t0 Duckweed/ Tenebrio larvae 24-31 85 ± 7 hours t0 Fish 20 89 ± 6; 115 ± 9 hours t0, MRT Fish 30 70 ± 3; 89 ± 51 hours t0, MRT Valisnaeria 20 260 ± 10; 310 ± 30 hours t0, MRT Vinyl tape Valido and Nogales (2003)69 Waldschmidt et al. (1986) 9 Kummrow et al. (2011) 26 Staton et al. (1992)3 5x5 mm 20 Valisnaeria 30 118 ± 8; 158 ± 12 hours t0, MRT Fish 20 154 ± 12 hours MRT Fish 30 123 ± 11 hours MRT Valisnaeria 20 418 ± 25 hours MRT Valisnaeria 30 180 ± 12 hours MRT Adult crickets, Coleoptera larvae, Plocama endula, Solanum lycopersicum, Solanaceae 28-30 6,9 ± 3,8 days tmax Gallotia atlantica (Teno Bajo) Omnivore Glass beads 3mm in diameter 0,02g 2 Mixed in food, force fed Gallotia atlantica (Izaña) Omnivore Glass beads 3mm in diameter 0,02g 2 Mixed in food, force fed 28-30 6,1 ± 5,3 days tmax Gallotia galloti (Tetir) Omnivore Glass beads 3mm in diameter 0,02g 2 Mixed in food, force fed 28-30 2,4 ± 1,5 days tmax Uta stansburiana Insectivore Polyacrylamide beads 100 µm in diameter Unknown Injected in cricket Acheta sp. 22, 24, 28, 32, 36 - t0 Chamaeleo calyptratus Insectivore Glitter <1 mm in diameter Live crickets coated in honey and glitter Acheta sp. 26-30 4 days tmod Alligator mississippiensis Carnivore Chromic oxide Voluntarly fed Experimental diet 28/32 Unknown 0,1% of the diet Davenport et al. (1990) 36 Crocodylus porosus Carnivore Chromic oxide Unknown minced with fresh fish (2% by weight) Sprattus 30 68h;92-113h t0; tmax Crocodylus porosus Carnivore Barium sulphate Unknown Minced fish with barium sulphate (20% by weight) Sprattus 30 see article for detailed gut movement tV * 100 Minced fish with barium sulphate (10% by weight), beads mixed in 2 g of fish Sprattus 30 - Barium sulphate 0,3 ml Injected in whole fish and live crabs Sprattus and crab 30 66 h Barium polysterene spheroids/ barium sulphate Davenport et al. (1992) 37 Tracy et al. (2006) 44 1 mm in diameter Caiman crocodylus Carnivore Chromic oxide Unknown Mixed in minced ox liver (2% w/w) Minced Ox liver 30 136 h tmax Caiman crocodylus Carnivore Barium sulphate Unknown Mixed in minced ox liver (2% w/w) Minced Ox liver 30 >12 h tV * Gopherus agassizii Herbivore Barium polystyrene spheroids 1 mm in diameter Unknown Mixed in minced ox liver (2% w/w) Minced Ox liver 30 - Lead glass beads 0.4 mm in diameter Unknown Mixed in minced ox liver (2% w/w) Minced Ox liver 30 - Ytterbium (Yb) Yb for fiber fraction Guinea pig chow, chick-starter chow 30 13 days t50 Cobalt (CoEDTA) Co-EDTA for liquid fraction 30 7 days t50 Barboza (1995)10 Xerobates agassizii Herbivore Chromic oxide Ytterbium (Yb) Cr -mordanted Cobalt (CoEDTA) Franz et al. (2011)70 Testudo graeca Herbivore Cr -mordanted Cobalt (CoEDTA) 150 mg Cr in 1,5 g dose 3 mg Yb in 1,5 g dose 38 mg Cr in 1,5 g dose 62 mg Co Placed into the back of the mouth by a syringe Placed into the back of the mouth by a syringe Placed into the back of the mouth by a syringe Single intra-gastric dose Labelling particles <2mm Herbage 31 9,57 ± 1,48; 9.55 ± 1.31 days Low-fiber pellet 31 5,29 ± 0.01; 6.73 ± 0.22 days Grass 31 5,2 ± 1,38; 9.60 ± 1.06 days High-fiber pellet 31 9,66 ± 3,75; 13.25 ± 3.09 days Grass 31 14,95 ± 2,36; 14.22 ± 1.83 days High-fiber pellet 31 12,67 ± 5,56; 14.77 ± 4.80 days Grass 31 4,81 ± 1,84; 6.78 ± 0.82 days High-fiber pellet 31 6,92 ± 3,60; 7.80 ± 3.82 days Grass hay/ lettuce 27-30 89-238 hours Grass hay/ lettuce 27-30 - tmax; MRT Range of MRT Testude hermanni Herbivore Cr -mordanted Labelling particles <2mm Cobalt (CoEDTA) Geochelone nigra Herbivore Cr -mordanted Labelling particles <2mm Cobalt (CoEDTA) Geochelone sulcata Herbivore Cr -mordanted Labelling particles <2mm Cobalt (CoEDTA) Draco dussumieri Herbivore Cr -mordanted Labelling particles <2mm Cobalt (CoEDTA) Miller et al. (2012) 7 Trachylepis margaritifer Insectivore Glass beads 2 per kricket Implementation in dead kricket Grass hay/ lettuce 27-30 90-150 hours Grass hay/ lettuce 27-30 - Grass hay/ lettuce 27-30 131-197 hours Grass hay/ lettuce 27-30 65-139 hours Grass hay/ lettuce 27-30 209-556 hours Grass hay/ lettuce 27-30 99-340 hours Grass hay/ lettuce 27-30 202-210 hours Grass hay/ lettuce 27-30 125-149 hours Acheta domstica 25 53,52 ± 21,00 hours 27 61,89 ± 10,69 hours 32 22,47 ± 12,4 hours tII – tV ** Barium sulphate 1:1 BaSO4/H20 supsension, 0,15 ml per kricket Barium sulphate Valente et al. (2008) 1:1 BaSO4/H20 supsension, 0,15 ml per kricket Caretta caretta Carnivore Coloured dishes of flat foam 5 mm in diameter Caretta caretta Carnivore BIPS® capsules 1,5 mm (30U), 5 mm (10U) 5 mm in diameter 29 Coloured dishes of flat foam 10-20 Injected in dead kricket Injected in dead kricket Placed inside fish at first feeding Placed inside fish at first feeding 10-20 Placed inside fish at first feeding Acheta domstica Acheta domstica Merluccius merluccius/ Sardina pilchardus sardina 25 49,47 ± 7,76 hours 27 56,46 ± 13,00 hours 32 16,21 ± 7,76 hours 25 21,75 ± 4,17 hours 27 31,85 ± 8,57 hours 16,2723,86 9,05 ± 3,05; 12,00 ± 4,53; 13,19 ± 4,64 days t0; t50; t85 16,2723,86 13,25 ± 4,86 days t0 8,5 ± 2,73 days t0 * tV = Time at which the digesta reached the rectum ** tII – tV = The time from when gastric emptying was initiated until movement into the large intestine was complete (phase II through V) was defined as the intestinal passage time Legend Pigments and dyes Particulate markers Chemical markers Radiopaque markers