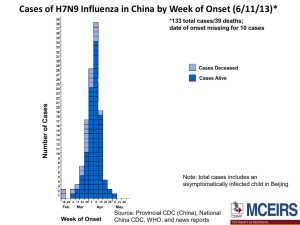
2014-04-03 Surveillance update
advertisement
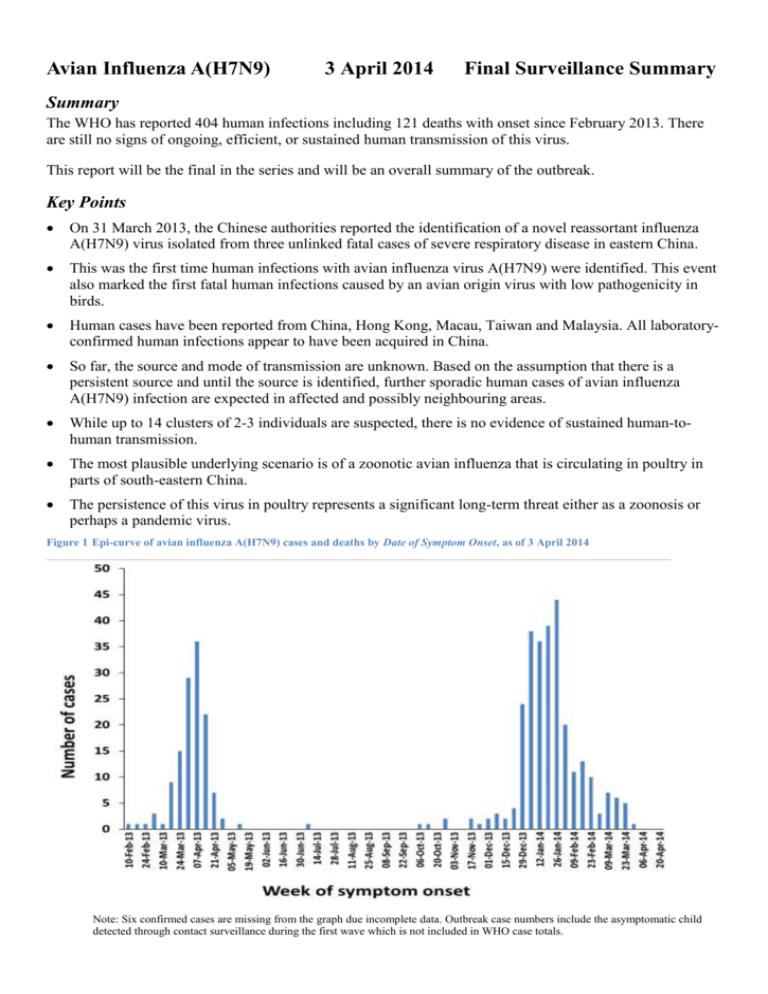
Avian Influenza A(H7N9) 3 April 2014 Final Surveillance Summary Summary The WHO has reported 404 human infections including 121 deaths with onset since February 2013. There are still no signs of ongoing, efficient, or sustained human transmission of this virus. This report will be the final in the series and will be an overall summary of the outbreak. Key Points On 31 March 2013, the Chinese authorities reported the identification of a novel reassortant influenza A(H7N9) virus isolated from three unlinked fatal cases of severe respiratory disease in eastern China. This was the first time human infections with avian influenza virus A(H7N9) were identified. This event also marked the first fatal human infections caused by an avian origin virus with low pathogenicity in birds. Human cases have been reported from China, Hong Kong, Macau, Taiwan and Malaysia. All laboratoryconfirmed human infections appear to have been acquired in China. So far, the source and mode of transmission are unknown. Based on the assumption that there is a persistent source and until the source is identified, further sporadic human cases of avian influenza A(H7N9) infection are expected in affected and possibly neighbouring areas. While up to 14 clusters of 2-3 individuals are suspected, there is no evidence of sustained human-tohuman transmission. The most plausible underlying scenario is of a zoonotic avian influenza that is circulating in poultry in parts of south-eastern China. The persistence of this virus in poultry represents a significant long-term threat either as a zoonosis or perhaps a pandemic virus. Figure 1 Epi-curve of avian influenza A(H7N9) cases and deaths by Date of Symptom Onset, as of 3 April 2014 Note: Six confirmed cases are missing from the graph due incomplete data. Outbreak case numbers include the asymptomatic child detected through contact surveillance during the first wave which is not included in WHO case totals. The first wave of human A(H7N9) infections involved 136 cases including 44 deaths with onset from 19 February to 30 September 2013. Cases were reported from 12 regions in mainland China. Only a few cases of human influenza A(H7N9) were reported over the summer months. The second wave of A(H7N9) infections which started in October 2013 is ongoing and has so far involved an additional 268 cases including 77 deaths. The re-emergence of human infections with A(H7N9) may be an indicator of an enlargement of the reservoir, an increase in the number of exposed individuals, enhanced transmissibility of the virus, a seasonal transmission pattern, or any combination of these factors. In this report, wave 1 cases include those with reported date of onset between 19 February and 30 September 2013. Wave 2 includes those with onset from 1 October 2013 to 23 March 2014 (Table 1). Figure 2 Map of human cases of avian influenza A(H7N9), by province, China, to 3 April 2014. Source: Center for Infectious Disease Research and Policy, University of Minnesota. Note: Chinese provinces with reported cases are shaded in blue. Countries with imported cases are shaded in green. This figure includes cases and deaths confirmed by local authorities; some of these cases have not yet been acknowledged by WHO. Table 1 Summary of avian influenza A(H7N9) laboratory-confirmed human cases and deaths by region and season. Region of diagnosis Anhui Beijing Fujian Guangdong Guangxi Hebei Wave 1 cases (deaths) 4(3) 3(0) 5(0) 1(0) 1(1) Wave 2 cases* 7 2 17 93 4 - Region of diagnosis Henan Hong Kong SAR Huangyan Hunan Jiangsu Jiangxi Jilin Malaysia Shandong Shanghai Zhejiang Taiwan Wave 1 cases (deaths) 4(1) 2(1) 23(8) 6(2) 2(0) 32(15) 46(8) - Wave 2 cases* 7 1 17 20 1 1 10 93 2 *Deaths in wave two have not been routinely reported with case information which has prohibited accurate case/death data matching. Clinical aspects and spectrum of disease Accurate determination of the incubation period is difficult due to a lack of poultry exposure data. The latest published analysis indicates a mean incubation period of 3.1 days (95% CI 2·6–3·6, SD 1·4 days, 95th percentile 5·5 days) derived from 32 cases with information about dates of recent exposures to live poultry [1]. Most of the cases have presented with influenza-like illness, with a minority reporting diarrhoea or vomiting [2]. Unlike other avian influenza A(H7) infections, initial conjunctivitis has not been a reported feature [3]. The disease caused by the virus is characterised by rapidly progressing severe pneumonia. Common symptoms are not disease specific and include those of typical acute respiratory infection, such as fever, cough, and shortness of breath. Complications include the acute respiratory distress syndrome (ARDS), septic shock and multi-organ failure requiring intensive care and mechanical ventilation. A small number of patients with mild clinical illness have been detected through on-going influenzalike illness (ILI) surveillance systems and contact tracing in otherwise healthy children and young adults. A detailed analysis of the epidemiologic characteristics of avian influenza A(H7N9) cases in China to 1 Dec 2013[4] has indicated that o Among 139 persons with confirmed A(H7N9) virus infection, 71% were male, and 73% were urban residents (Figure 3). o Nine persons were poultry workers, and of 131 persons with available data, 82% had a history of exposure to live animals, including chickens (82%). o A total of 137 persons (99%) were hospitalised, 125 (90%) had pneumonia or respiratory failure, and 65 of 103 with available data (63%) were admitted to an intensive care unit. o A total of 47 persons (34%) died in the hospital after a median duration of illness of 21 days, 88 were discharged from the hospital, and 2 remain hospitalised in critical condition; 2 patients were not admitted to a hospital. o The estimated case-fatality risk is 36% on admission to hospital among the first 123 cases and between 0.16–2.8% of the symptomatic cases in the community [5]. Health status (where reported) on hospital admission was not significantly different between waves (p=0.11 Χ2 test) ( Table 2). Table 2 Summary of health status on hospital admission of avian influenza A(H7N9) laboratory-confirmed human cases by season. Hospital Admissionhealth status Mild Serious Critical Wave 1 (n=95) Wave 2 (n=230) 4% (4 cases) 22% (21 cases) 74% (70 cases) 6% (12 cases) 30% (70 cases) 64% (148 cases) The period between symptom onset and hospitalisation was also similar between wave 1 and wave 2 cases (wave 1 median 4 days, range 0-11; wave 2 median 5 days, range 0-14) suggesting that the rate of disease progression has not changed. To help inform the proportion of asymptomatic infections serological studies were conducted among Chinese poultry workers and healthcare workers. In the first study, 126 healthy healthcare workers and 615 healthy non-healthcare workers were tested for the presence of A(H7N9) antibodies: a single serum sample from each group contained a high level of H7-specific antibodies [6]. In another study, 6% (25/396) of poultry workers had elevated titres for antibodies specific for avian-origin A(H7N9) virus versus none of 1,129 samples tested in the general population [7]. Compared to seasonal influenza which has an estimated asymptomatic fraction of approximately 77% [8], these results confirm the elevated severity of A(H7N9) infection and suggest that the majority of cases will be identified through hospital based surveillance. Figure 3 Distribution of laboratory confirmed avian influenza A(H7N9) cases by age and sex (n=404) Current Treatment Recommendations The current treatment practices do not differ from treatment of other severe influenza disease. WHO recommends immediate empirical treatment of symptomatic individuals exposed to A(H7N9) with neuraminidase inhibitors. Chemoprophylaxis of asymptomatic exposed individuals is not recommended [9]. The US CDC has also published interim guidance on the use of antivirals for A(H7N9) [10]. The WHO Collaborating Centre in Beijing has confirmed that influenza A(H7N9) virus is sensitive to oseltamivir and zanamivir in phenotypic tests [2]. However, Arg292Lys substitutions in the neuraminidase gene which are associated with resistance to neuraminidase inhibitors, have been documented in several treated cases and tend to lead to a poor clinical outcome [11]. Surveillance and Travel Advice WHO advises that travellers to countries with known outbreaks of avian influenza should avoid poultry farms, or contact with animals in live bird markets, or entering areas where poultry may be slaughtered, or contact with any surfaces that appear to be contaminated with faeces from poultry or other animals. Travellers should also wash their hands often with soap and water. Travellers should follow good food safety and good food hygiene practices Heightened vigilance of respiratory infections in humans at clinical and jurisdictional levels, and rapid reporting of cases infected by A(H7N9) is encouraged. Animal infections and virus detection in environmental specimens Active surveillance is ongoing in China, where public health authorities sample chickens, waterfowl, captive-bred pigeons, quails and wild birds. Additionally, environmental samples are collected at wholesale live bird markets, live bird trading areas (stalls) at farmers' markets, large-scale poultry farms, village/backyard poultry holdings, poultry slaughterhouses, wild migrating bird habitats, and other locations. Genetically similar influenza A(H7N9) isolates have so far been detected in ducks, pigeons and chickens, but it has not been detected in pigs [12, 13]. Goose meat has also tested positive for influenza A(H7N9). Animal Health and Control Measures A significant difference between influenza A(H5N1) and A(H7N9) avian influenza viruses is the reduced pathogenicity of the A(H7N9) virus in poultry. H5N1 is highly pathogenic in poultry and can be detected by flock die-offs. Influenza A(H7N9) does not severely affect poultry, and it is likely that influenza A(H7N9) can circulate silently in poultry and other bird populations. The human cases may be the first indication of infections in birds. The major source of infection for humans seems to be poultry handled in the poultry market [14], while wild birds are the reservoir for H7 and N9 genes of influenza viruses. The Ministry of Agriculture reported that ‘stamping-out’ control measures, including periodic market closures, have been implemented in poultry markets where A(H7N9) avian influenza viruses have been detected. These closures were associated with a decrease in the number of human cases in those localities [15, 16]. Outstanding Issues Further epidemiological investigations are needed in order to identify risk behaviours, other risks, and predisposing factors for avian influenza A(H7N9) infection. Whether there is a role for influenza A(H7N9) vaccines for poultry in affected areas also requires consideration. If the A(H7N9) virus manages to spread widely in poultry without detection and becomes highly pathogenic in poultry, food security in China might become a significant concern [17]. Laboratory studies in animals suggest there are reasons for further concern over the pandemic potential of influenza A(H7) viruses in general [18, 19]. A recent report described an evolutionary dynamics model for acquisition of efficient human-to-human transmission via mutations. The authors estimated that if the virus continues to mutate at the current rate, the virus would require a further 11.3 years (95% confidence interval 11.2-11.3) to acquire properties for human-to-human transmission [20]. (Moderators comment – It is interesting to note that the authors did not attempt to test their methodology using A(H5N1) which is essentially the experiment to test the model that has been running for 17 years.) A report compiled by Dr Rachel de Kluyver, Health Emergency Management Branch, Australian Government - Department of Health. email rachel.de.kluyver@health.gov.au References 1. 2. 3. 4. 5. 6. Cowling, B.J., et al., Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. The Lancet, 2013. 382(9887): p. 129-137. Gao, R., et al., Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med, 2013. 368(20): p. 1888-97. Li, Q., et al., Preliminary Report: Epidemiology of the Avian Influenza A (H7N9) Outbreak in China. N Engl J Med, 2013. 24: p. 24. Li, Q., et al., Epidemiology of Human Infections with Avian Influenza A(H7N9) Virus in China. New England Journal of Medicine, 2014. 370(6): p. 520-532. Yu, H., et al., Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet, 2013. 382(9887): p. 138-45. Xu, W., et al., Serological investigation of subclinical influenza A(H7H9) infection among healthcare and non-healthcare workers in Zhejiang Province, China. Clin Infect Dis., 2013. 57(6): p. 919-21. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Yang, S., et al., Avian-origin H7N9 virus infection in H7N9-affected areas of China: a serological study. Journal of Infectious Diseases, 2013. Hayward, A.C., et al., Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. The Lancet Respiratory Medicine, 2014. World Health Organization Avian influenza A(H7N9) virus: Post-exposure antiviral chemoprophylaxis of close contacts of a patient with confirmed H7N9 virus infection and/or high risk poultry/environmental exposures. Avian influenza A(H7N9) virus 2014. 2. Lu, S., et al., Clinical Findings for Early Human Cases of Influenza A(H7N9) Virus Infection, Shanghai, China. Emerg Infect Dis, 2013. 19(7): p. 130612. Hu, Y., et al., Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet, 2013. 381(9885): p. 2273-9. Feng Y, M.H., Xu C, Jiang J, Chen Y, Yan J, et al. , Origin and characteristics of internal genes affect infectivity of the novel avian-origin influenza A(H7N9) virus. PloS one, 2013. 8(11):e81136. China, M.o.A.o.t.P.s.R.o. H7N9 found in south China poultry market 2014. 2014 [cited 2014 3/4/2014]; Available from: http://english.agri.gov.cn/rone/201401/t20140107_20988.htm. Ding, H., et al., Epidemiologic characterization of 30 confirmed cases of human infection with avian influenza A(H7N9) virus in Hangzhou, China. BMC Infectious Diseases, 2014. 14(1): p. 175. World Health Organisation. Transcript of press conference in Beijing - The China-WHO Joint mission on H7N9 assessment. 2013 24 April 2013 [cited 2014 3/4/2014]; Available from: http://www.wpro.who.int/china/topics/h7n9_influenza/transcript_20130424/en/. Murhekar, M., et al., Avian influenza A(H7N9) and the closure of live bird markets. Western Pac Surveill Response J., 2013 4(2): p. 4-7. Zhuang, Q., et al., Epidemiological and risk analysis of the H7N9 subtype influenza outbreak in China at its early stage. Chinese Science Bulletin, 2013. 58(26): p. 3183-3187. Belser, J.A., et al., Pathogenesis, transmissibility, and ocular tropism of a highly pathogenic avian influenza A (H7N3) virus associated with human conjunctivitis. J Virol, 2013. 87(10): p. 5746-54. Nicoll, A. and N. Danielsson, A novel reassortant avian influenza A(H7N9) virus in China - what are the implications for Europe. Euro Surveill., 2013. 18(15): p. 20452. Peng J, et al., The Origin of Novel Avian Influenza A (H7N9) and Mutation Dynamics for Its Human-To-Human Transmissible Capacity. Plos one, 2014. 9(3): p. e93094.