Mike Shaw - Institute for People and Technology
advertisement

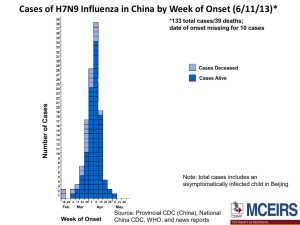
Computational Challenges for Infectious Diseases Michael Shaw, PhD OID/Office of the Director Office of Infectious Diseases CDC’s Scientific Agenda for AMD: To use modern laboratory and computing technologies to enhance public health surveillance, response to outbreaks, and the control and prevention of infectious diseases Pathogen Detection and Characterization Applications. Molecular detection as a replacement for traditional methods such as culture/isolation or visualization of antigens/antibodies: Allows more laboratories to detect pathogens and thus increases the amount of surveillance data. Allows surveillance of more pathogens. Makes true Molecular Epidemiology possible. Challenge: Hepatitis C virus (HCV) exists in infected host as a large population of genetically related intra-host variants - A single sequence cannot adequately represent the intra-host viral population It is important to sample numerous intra-host viral variants for many molecular epidemiological applications: - detection of transmission networks - drug resistance - vaccine escape - disease severity Detection of HCV transmissions using NGS Clinical Institution HCV cases Detection of epidemiological links Surveillance Next-Generation Sequencing Computational tools Not linked Most probable source Network of transmissions Transmission cluster Ganova-Raeva, L. et al. Detection of hepatitis C virus transmission using mass spectrometry. Journal of Infectious Diseases. 207(6):999-1006. Challenge: NGS error correction Challenge • Distinguishing viral variants from NGS errors • Extremely large data sets - Blue dot represents the only real variant - Yellow dots are NGS errors Solution • Error correction algorithms Skums, P. Et al. Efficient Error Correction of High-throughput Viral Sequencing. 2011. BMC bioinformatics. 2012, 13(Suppl 10):S6. Challenge: Risk Assessment of an Emerging Pathogen, Influenza A (H7N9) Hemagglutinin Structure RBS AS-B AS-A AS-D AS-E AS-C Antigenic Site A Red Antigenic Site B Gold Antigenic Site C Magenta Antigenic Site D Cyan Antigenic Site E Green Receptor Binding Site Gray Equivalent sites to H3N2 viruses: Wiley et al. 1981, Nature 289:373 Popova et al. 2012, PLoS One 7:e421895 Daniels et al. 1983, J Gen Virol 64:1657 Stray et al. 2012, Virol J 9:91 H7N9: Genetic Markers Characteristic of Host Adaptation or Virulence • NA stalk deletion aa 69-73 characteristic of poultry adaptation • M1 protein: N30D and T215A – increased virulence in mice • PB2: • 89V – enhanced polymerase activity and increased virulence in mice • 627K - enhanced polymerase activity and increased virulence in mice (most human isolates; absent in avian or environmental virus sequences) • PB1: • H99Y and I368V – H5 transmissibility in ferrets; not present in all • NS1 • P42S – increased virulence in mice H7 Receptor binding site Netherlands/219/2003 vs 2013 H7N9 Minimal impact, if any on the RBS. Assuming receptor binding is similar to published structural data, this should not directly interact with receptor 177: V / G / G / V Point towards the RBS pocket. More hydrophobic in Anhui/1/2013 May reduce α2-3 interactions? 212: T 125: A / T / A / A P, conserved in other H7 HAs Glycosylation site at position 123 in NL219 is not present in 2013 H7N9 217: L / Q / Q / I Equivalent to residue 226 in H3 numbering. Crucial for switching between α2-3 and α2-6 receptor specificity in H2/H3 HAs. 180: A / T / A / A 128: S A, conserved in other H7 HAs Glycans and influenza virus specificity A/Netherlands/219/2003 (H7N7) Avian Receptor-binding Pattern A/Anhui/1/2013 (H7N9) A/New Caledonia/20/1999 (pre2009 H1N1) Seasonal Human Pattern a2-3 Avian-type receptors, found in human lower respiratory tract a2-6 Human-type receptors, found in human upper respiratory tract Outbreak Response • Detection of etiologic agent – Identification of previously unknown pathogens • SARS and MERS CoV – Distinguish from background of commensals – Increasing reliance on PCR and sequencing • Characterization of etiologic agent – Tissue tropism and host range • Clinical recognition and management • Non human reservoir identification (important for control efforts) – Diagnostics development – Susceptibility to antimicrobial therapeutics – Vaccine development and use Questions? • Michael Shaw, Office of Infectious Diseases – MShaw1@cdc.gov • Yuri Khudyakov, Division of Viral Hepatitis – YKhudyakov@cdc.gov





![Shark Electrosense: physiology and circuit model []](http://s2.studylib.net/store/data/005306781_1-34d5e86294a52e9275a69716495e2e51-300x300.png)




