Click Here to
advertisement
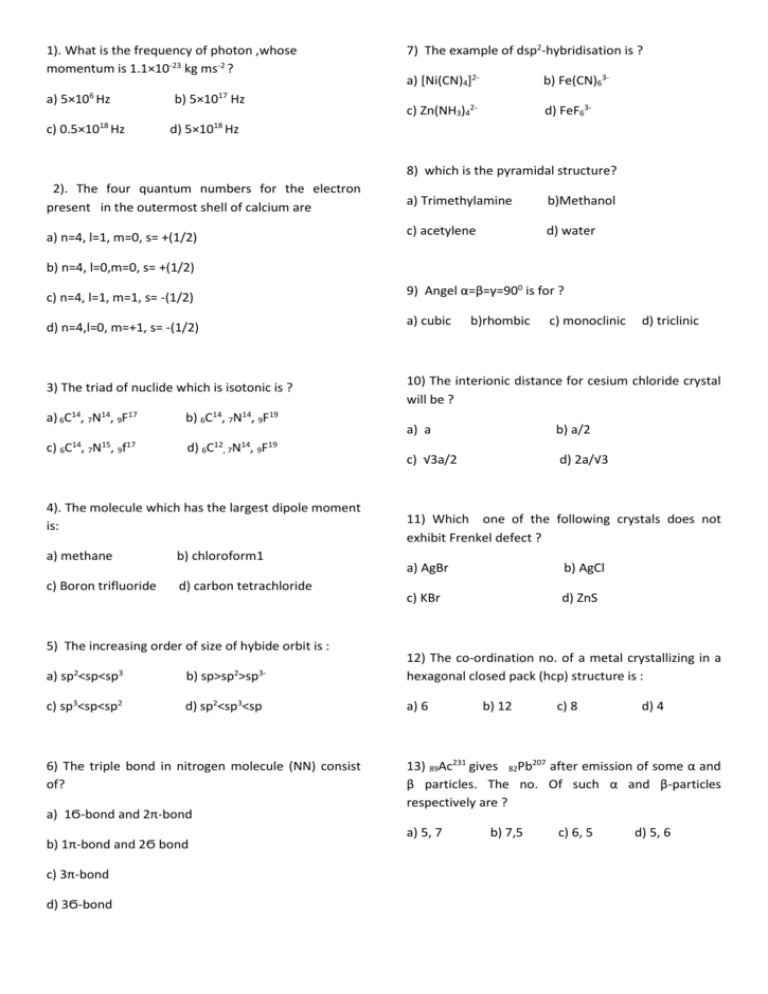
1). What is the frequency of photon ,whose momentum is 1.1×10-23 kg ms-2 ? a) 5×106 Hz b) 5×1017 Hz c) 0.5×1018 Hz 7) The example of dsp2-hybridisation is ? a) [Ni(CN)4]2- b) Fe(CN)63- c) Zn(NH3)42- d) FeF63- d) 5×1018 Hz 8) which is the pyramidal structure? 2). The four quantum numbers for the electron present in the outermost shell of calcium are a) Trimethylamine b)Methanol a) n=4, l=1, m=0, s= +(1/2) c) acetylene d) water b) n=4, l=0,m=0, s= +(1/2) c) n=4, l=1, m=1, s= -(1/2) 9) Angel α=β=γ=900 is for ? d) n=4,l=0, m=+1, s= -(1/2) a) cubic 3) The triad of nuclide which is isotonic is ? 10) The interionic distance for cesium chloride crystal will be ? a) 6C14, 7N14, 9F17 14 15 17 c) 6C , 7N , 9f b) 6C14, 7N14, 9F19 d) 12 14 19 6C , 7N , 9F 4). The molecule which has the largest dipole moment is: a) methane b) chloroform1 c) Boron trifluoride d) carbon tetrachloride 5) The increasing order of size of hybide orbit is : 2 a) sp <sp<sp 3 c) sp3<sp<sp2 2 b) sp>sp >sp 3- d) sp2<sp3<sp 6) The triple bond in nitrogen molecule (NN) consist of? a) 1Ϭ-bond and 2π-bond b) 1π-bond and 2Ϭ bond c) 3π-bond d) 3Ϭ-bond b)rhombic c) monoclinic a) a b) a/2 c) √3a/2 d) 2a/√3 d) triclinic 11) Which one of the following crystals does not exhibit Frenkel defect ? a) AgBr b) AgCl c) KBr d) ZnS 12) The co-ordination no. of a metal crystallizing in a hexagonal closed pack (hcp) structure is : a) 6 b) 12 c) 8 d) 4 13) 89Ac231 gives 82Pb207 after emission of some α and β particles. The no. Of such α and β-particles respectively are ? a) 5, 7 b) 7,5 c) 6, 5 d) 5, 6 20) In the reaction, 2A+B→A2B, if the concentration of A is doubled and of G is halved, then the rate of the reaction will ? 14) In nuclear reaction , 14 4 7N +2He z XA+1H2 The term zXA represent : a) increase by two times a) 7N16 b) decrease by three times b) 8O16 c) 7N15 d) 8O17 c) increase by four times d) remain the same 15) In the thorium series 90Th232 loses total of 6αparticle and 4β-particle in the ten stages the final isotope produced in the series is : a) 32Pb206 b) 83Pb208 c) 83Bi209 d) 82Pb209 21) Think for this reaction 2A+B → C+D. If the conc. Of reactant increase 3 times then the velocity ? a) increases 27 times b) increases 9 times 16) Which of the following exhibits highest molar conductivity ? c) increases 81 times d) increases 18 times a) Co(NH3)3Cl2 b) [Co(NH3)4Cl2]Cl 22) H2+Cl2 → 2HCl is ? c) [Co(NH3)5Cl]Cl2 a) first order reaction d) [Co(NH3)6]Cl3 b) second order reaction c) third reaction 17) What will be the molarity of a solutions containing 5g of sodium hydroxide in 250 mL solution ? a) 0.5 b) 1.0 c) 2.0 d) 0.1 18) 0.126 of ac acid requires 20 mL of 0.1 N NAOH for complete neutralization .The equivalent weight for the acid is ? a) 63 b) 53 c) 40 d) zero order reaction 23) For the first order reaction A→ product the halflife time is 200 s. The rate constant of the reaction is ? a) 3.46 s-1 b) 3.46× 10-3 1-1 c) 3.46 × 10-4 s -1 d) 3.46 × 10-2 s-1 d) 45 24) What condition will favour theexothermic ammonia synthesis reaction ? 19) The isomotic pressure of 0.2 molar solution of urea at 270 C (R=0.082L atm mol-1K-1) ? a) 4.92 b) 1 atm c) 0.2 atm d) 27 atm N2(g)+3H2(g) a) b) c) d) 2NH3(g) Low temperature and low pressure Low temperature and high pressure High temperature and low pressure High temperature and high pressure 25) For the reaction PCl5(g) PCl3(g)+ Cl2(g) 30) Given that the dissociation constant for H2O is Kw=1×10-14 .what is the pH of a 0.001 molar KOH solution? a) Kp = Kc (RT) b) Kp = Kc (RT) a). 11 c) Kp = Kc d) Kp = Kc (RT) c) 10-11 26) The favourable condition for contact process are ? a) low temperature and low temperature b) low temperature and high pressure c) high temperature and low pressure b) 14 d)3 31) The enthalpy change for the conversion of liquid water to stream at 373 is ΔHvap=37.3 kJ mol-1 .The entropy change for the process is? a) 100 J mol-1 K-1 b)74.6 mol-1 K-1 c) 37.3 J mol-1 k-1 d) 111.9 J mol-1 k-1 d) high temperature and high pressure 32) Human body is the example of : 27) For the reaction : a) open system PCL3 (g)+ Cl2(g) b) closed system PCl5(g) c) isolated system d) none of these The positionn of equilibrium can be shifted to the right way a) doubling the volume 33) Heat of neutralization will be minimum for which of the following combination? b) increasing the temperature a)NaOH+H2SO4 b)NH4OH+CH3COOH c) addition of equimolar quantities of PCl3 and PCl5 c) NAOH+HCL d) NAOH+CH3COOH d) addition of Cl2 at constant volume 28). The solubility product of Mg(OH)2 at 250C 1.4×10-11 What is the solubility of Mg(OH)2 in g/L ? a) 0.0087g/L b)0.087g/L c) 0.0047 g/L d) 0.047g/L 34) Heat of formation of SO2 is -298 kJ.what is heat of combustion of 4 g of S? a)+37kJ b)-37.25kJ c)+298 kJ d)18.6 kJ 35) Oxidation state +1 for phosphorous is found in : 29) The heat of neutralization of a strong acid and strong base is nearly equal to ? a) H4P2O7 b) H3PO2 c) H3PO4 d) H3PO3 a) -13.7 kcl/mol b)+13.7 kcl/mol c) +57.32 kcl/mg d) _57.32kcl/mol 36) The Oxidation number of nitrogen in NO3- is: a) +5 b) +2 c) -1 d) +3 37) Oxidation state of S in H2S2O8 is: a) +2 b) +4 c) +6 d) +7 44) colour of the colloids depend on which of the following factors ? a) Size b) Mass c) Charge d) Nature 38) Pressian blue is : a) Fe[Fe(CN)6]2 b) Fe3[Fe(CN)6] 45) The correct order of the solubility of sulphates of alkaline earth metal in water is : c) Fe2[Fe(CN)6] d) Fe4[Fe(CN)6]3 a) Be>Ca>Mg>Ba>Sr b) Mg>Be>Ba>Ca>Sr 39) Which of the following complex shows ionization isomorphism ? a) [Cr(Co)6] b) [Pt(NH3)2Cl2] c) [Cr(NH3)3Cl3 ] c) Be>Mg>Ca>Sr>Ba d) Mg>Ca>Ba>Be>Sr d) [Cr(en)2Cl2] 46) which of the following has smallest size ? 40) The IUPAC name of K3[Fe(CN)6] is : a) N3- b) O2- d) Na+ c) F a) potassium hexa cynoferrate (III) b) potassium ferro hexacyanate(II) 47)Which of the following is correct order of the size of the iodine species ? c) potassium hexa ferrocyanate(III) a) I >I- >I+ d) potassium ferrocyanide (II) c) I+ >I- >I 41) The coordination number of Cu in [Cu(H2O4)]2+ complex 48) which of the following has largest radius ? a) 2 b) 1 c) 3 d) 4 42) In the given reaction , As2o3 act as a : 2SO2+ O2 Pt 2SO3 a) F- b) I >I+ >Id) I- >I >I+ b) O2- c) Na+ d) Mg2+ 49)The lightest metal among these is : a) Li b) Mg c) Ca d) Na As2O3 a) Positive catalyst b) promoter c) autocatalyst d) poison 50) Nessler’s reagent is: 43) Which of the following cannot form the micelles ? a) KHgl4+NH4OH a) sodium benzoate c) K2Hgl4+KOH b) KHgl4+KOH d) KHgl4 b) sodium loryl sulphate c) sodium alkyl benzene sulphonate 51) which of the metal is preserved in water ? d) sodium oliate a) Platinum b) sodium c) zinc d) Iron 52) which of the following is formula of baking powder ? 59) AgCl precipitate dissolves in ammonia due to the formation of : a) NaHCO3 a) [Ag(NH3)2]Cl b)[Ag(NH3)2]OH c) [Ag(NH4)2]Cl d) [Ag(NH4)2]OH b) NaNO3 c) NaOH d) NaCl 53) The correct sequence of decrease in the bond angle of the following hydride is : a) NH3>PH3>AsH3> SbH3 60) In borax bead test, deep blue colour bead is obtained due to : b) NH3>AsH3>PH3>SbH3 a) (C2H5)3 BO3 b) H3 BO4 c) SbH3>AsH3>PH3>NH3 c) (C2H5)2 B4O7 d) H2B4O7 d) PH3>NH3>AsH3>SbH3 61) Which of the following is observed by the chromyl chloride test ? 54) In photography we use: a) Agl b) NH3 c)AgCl a) Cl- d) AgBr 55) The composition of bell metal is : a) Cu+Sn b) Cu+ Ni c) Cu+ Zn d) Cu+Ag c) CuSO4. 5H2O c) I- CH2―CH―CH2 is: CN CN CN a) 1,2,3 tricyano propane b) 3-cyano-1,5 percent di nitrile c) 1,2,3-cyano propane d) None of the above b) ZnSO4.7H2O d) CuSo4. ½ H2O 63) The bond order of individual carbon-carbon bond is benzene is: a) 1 57) Which pair consist only acidic oxides ? a) Cro3, Mn 2O7 c) Cao,Zno d) Na2O, Al2O3 b) Co2+ b) 2 c) between 1 and 2 d) 1 and 2 alternately b) ZnO2, Al2O3 64)The number of ether isomers possible for C4H10O are : 58) Which one of the given transition metal ions is : A) Cu2+ d) F – 62) The IUPAC name name of 56) Green vitriol is : a) FeSo4. 7H2O b) Br- c) Cr3+ a) 2 B) 5 c) 4 d) 3 d) Zn2+ 65) The empirical formula of compound is CH2- one mole of this compound has a mass of 56 g.Its molecular formula is : a) C3H6 b) C4H8 c)CH2 d) C2H2 72)CH3Br+KCN(alc.) 66) The smallest bond length of C-H is: reduction X Y Na+C2H5OH a) ethyne b) ether c) ethane d) methane a) CH3CN b) C2H5CN c) C2H5NH2 d) CH3NH2 67) A gas decolourised alkaline KMnO4 solution ang give precipate with AgNO3 solution the gas is: a) methane 73) Cao+C b) ethene Heat A H20 Dil.H2so4 B C, HgSO4 c) acetylene d) ethane The last product is: 68) Anti-markownikoff’s addition is not observed in : a) butane-2 b) butane-2 c) propene d) pentene-1 a) CH3CHO b) C2H5OH c) C2H4 d) C2H5HSO4 74) Acetamide p O 2 5 4H A B Sl/HCL 69) Identify Z in the following series : C2H5 Alcoholic X Br 2 Y KCN a) CH3CH2NH 2 c) CH3CN b) CH3NH2 d) CH3COONH4 Z KOH 75) Lactic acid+conc.H2SO4 a) CN―CH2― CH2― CN b)Br― CH2 ―CH2― CH2CN c) Br― CH == CHCN Heat a) CH3COOH b)CH3CHO c)CH3CH2COOH d)Acrylic acid X, X is d) CH3 CH2CN 76) which of the following is natural polymer ? 160-170 c 70) X+ H2SO4 Ethylene, X is : dehydration a) Dacron b) Nylon c) Polyethene d) Starch a) (CH3)2CHCH2OH b) CH3CH2CH2OH 77) Nucleic acid is a polymer of : c) CH3OH a) nucleotides b) α-amino acids d) C2H5OH c) nucleosides d) Glucose 71) R X+Mg dry ether product a) alkyne b) alkene c) GR d) magnesium halide 78) Which carbohydrate has highest abundance in human blood ? a) D- glucose b) D-fructose c) sucrose d) Lactose 79) Which one of the following is an example of azodye ? a) Aniline yellow b) Alizarin c) Martius yellow d) Indigo 80) Which of the following ion is stronger Bronsted base? a) ClO- b) ClO-2 c) ClO-3 d) ClO-4