RP 5.P.2 Matter & Energy - NC Science Wiki
advertisement

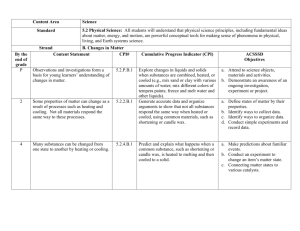
North Carolina Science Essential Standards 5.P.2 Resource Pack Essential Standard: 5.P.2 Understand the interactions of matter and energy and the changes that occur. 5.P.2.1 Explain how the sun’s energy impacts the processes of the water cycle (including evaporation, transpiration, condensation, precipitation and runoff). 5.P.2.2 Compare the weight of an object to the sum of the weight of its parts before and after an interaction. 5.P.2.3 Summarize properties of original materials, and the new material(s) formed, to demonstrate that a change has occurred. Vertical Strand Maps: http://scnces.ncdpi.wikispaces.net/Strand+Maps Online Atlas map http://strandmaps.dls.ucar.edu/?id=SMS-MAP-1325 http://strandmaps.dls.ucar.edu/?id=SMS-MAP-1341 http://strandmaps.dls.ucar.edu/?id=SMS-MAP-1349 North Carolina Unpacking: http://scnces.ncdpi.wikispaces.net/Race+to+the+Top+Support+Tools TEACHER KNOWLEDGE BLAST Framework for K-12 Science Education: Core Idea PS1 Matter and Its Interactions How can one explain the structure, properties, and interactions of matter? The existence of atoms, now supported by evidence from modern instruments, was first postulated as a model that could explain both qualitative and quantitative observations about matter (e.g., Brownian motion, ratios of reactants and products in chemical reactions). Matter can be understood in terms of the types of atoms present and the interactions both between and within them. The states (i.e., solid, liquid, gas, or plasma), properties (e.g., hardness, conductivity), and reactions (both physical and chemical) of matter can be described and predicted based on the types, interactions, and motions of the atoms within it. Chemical reactions, which underlie so many observed phenomena in living and nonliving systems alike, conserve the number of atoms of each type but change their arrangement into molecules. Nuclear reactions involve changes in the types of atomic nuclei present and are key to the energy release from the sun and the balance of isotopes in matter. PS1.A: STRUCTURE AND PROPERTIES OF MATTER How do particles combine to form the variety of matter one observes? While too small to be seen with visible light, atoms have substructures of their own. They have a small central region or nucleus—containing protons and neutrons—surrounded by a larger region containing electrons. The number of protons in the atomic nucleus (atomic number) is the defining characteristic of each element; different isotopes of the same element differ in the number of neutrons only. Despite the immense variation and number of substances, there are only some 100 different stable elements. Each element has characteristic chemical properties. The periodic table, a systematic representation of known elements, is organized horizontally by increasing atomic number and vertically by families of elements with related chemical properties. The development of the periodic table (which occurred well before atomic substructure was understood) was a major advance, as its patterns suggested and led to the identification of additional elements with particular properties. Moreover, the table’s patterns are now recognized as related to the atom’s outermost electron patterns, which play an important role in explaining chemical reactivity and bond formation, and the periodic table continues to be a useful way to organize this information. The substructure of atoms determines how they combine and rearrange to form all of the world’s substances. Electrical attractions and repulsions between charged particles (i.e., atomic nuclei and electrons) in matter explain the structure of atoms and the forces between atoms that cause them to form molecules (via chemical bonds), which range in size from two to thousands of atoms (e.g., in biological molecules such as proteins). Atoms also combine due to these forces to form extended structures, such as crystals or metals. The varied properties (e.g., hardness, conductivity) of the materials one encounters, both natural and manufactured, can be understood in terms of the atomic and molecular constituents presentand the forces within and between them. Within matter, atoms and their constituents are constantly in motion. The arrangement and motion of atoms vary in characteristic ways, depending on the substance and its current state (e.g., solid, liquid). Chemical composition, temperature, and pressure affect such arrangements and motions of atoms, as well as the ways in which they interact. Under a given set of conditions, the state and some properties (e.g., density, elasticity, viscosity) are the same for different bulk quantities of a substance, whereas other properties (e.g., volume, mass) provide measures of the size of the sample at hand. Materials can be characterized by their intensive measureable properties. Different materials with different properties are suited to different uses. The ability to image and manipulate placement of individual atoms in tiny structures allows for the design of new types of materials with particular desired functionality (e.g., plastics, nanoparticles). Moreover, the modern explanation of how particular atoms influence the properties of materials or molecules is critical to understanding the physical and chemical functioning of biological systems. Grade Band Endpoints for PS1.A By the end of grade 2. Different kinds of matter exist (e.g., wood, metal, water), and many of them can be either solid or liquid, depending on temperature. Matter can be described and classified by its observable properties (e.g., visual, aural, textural), by its uses, and by whether it occurs naturally or is manufactured. Different properties are suited to different purposes. A great variety of objects can be built up from a small set of pieces (e.g., blocks, construction sets). Objects or samples of a substance can be weighed, and their size can be described and measured. (Boundary: volume is introduced only for liquid measure.) By the end of grade 5. Matter of any type can be subdivided into particles that are too small to see, but even then the matter still exists and can be detected by other means (e.g., by weighing or by its effects on other objects). For example, a model showing that gases are made from matter particles that are too small to see and are moving freely around in space can explain many observations, including the inflation and shape of a balloon; the effects of air on larger particles or objects (e.g., leaves in wind, dust suspended in air); and the appearance of visible scale water droplets in condensation, fog, and, by extension, also in clouds or the contrails of a jet. The amount (weight) of matter is conserved when it changes form, even in transitions in which it seems to vanish (e.g., sugar in solution, evaporation in a closed container). Measurements of a variety of properties (e.g., hardness, reflectivity) can be used to identify particular materials. (Boundary: At this grade level, mass and weight are not distinguished, and no attempt is made to define the unseen particles or explain the atomicscale mechanism of evaporation and condensation.) By the end of grade 8. All substances are made from some 100 different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. Pure substances are made from a single type of atom or molecule; each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. Gases and liquids are made of molecules or inert atoms that are moving about relative to each other. In a liquid, the molecules are constantly in contact with each other; in a gas, they are widely spaced except when they happen to collide. In a solid, atoms are closely spaced and vibrate in position but do not change relative locations. Solids may be formed from molecules, or they may be extended structures with repeating subunits (e.g., crystals). The changes of state that occur with variations in temperature or pressure can be described and predicted using these models of matter. (Boundary: Predictions here are qualitative, not quantitative.) PS1.B: CHEMICAL REACTIONS How do substances combine or change (react) to make new substances? How does one characterize and explain these reactions and make predictions about them? Many substances react chemically with other substances to form new substances with different properties. This change in properties results from the ways in which atoms from the original substances are combined and rearranged in the new substances. However, the total number of each type of atom is conserved (does not change) in any chemical process, and thus mass does not change either. The property of conservation can be used, along with knowledge of the chemical properties of particular elements, to describe and predict the outcomes of reactions. Changes in matter in which the molecules do not change, but their positions and their motion relative to each other do change also occur (e.g., the forming of a solution, a change of state). Such changes are generally easier to reverse (return to original conditions) than chemical changes. “Collision theory” provides a qualitative model for explaining the rates of chemical reactions. Higher rates occur at higher temperatures because atoms are typically moving faster and thus collisions are more frequent; also, a larger fraction of the collisions have sufficient energy to initiate the process. Although a solution or a gas may have constant chemical composition—that is, be in a steady state—chemical reactions may be occurring within it that are dynamically balanced with reactions in opposite directions proceeding at equal rates. Any chemical process involves a change in chemical bonds and the related bond energies and thus in the total chemical binding energy. This change is matched by a difference between the total kinetic energy of the set of reactant molecules before the collision and that of the set of product molecules after the collision (conservation of energy). Some reactions release energy (e.g., burning fuel in the presence of oxygen), and others require energy input (e.g., synthesis of sugars from carbon dioxide and water). Understanding chemical reactions and the properties of elements is essential not only to the physical sciences but also is foundational knowledge for the life sciences and the earth and space sciences. The cycling of matter and associated transfers of energy in systems, of any scale, depend on physical and chemical processes. The reactivity of hydrogen ions gives rise to many biological and geophysical phenomena. The capacity of carbon atoms to form the backbone of extended molecular structures is essential to the chemistry of life. The carbon cycle involves transfers between carbon in the atmosphere—in the form of carbon dioxide—and carbon in living matter or formerly living matter (including fossil fuels). The proportion of oxygen molecules (i.e., oxygen in the form O2) in the atmosphere also changes in this cycle. Grade Band Endpoints for PS1.B By the end of grade 2. Heating or cooling a substance may cause changes that can be observed. Sometimes these changes are reversible (e.g., melting and freezing), and sometimes they are not (e.g., baking a cake, burning fuel). By the end of grade 5. When two or more different substances are mixed, a new substance with different properties may be formed; such occurrences depend on the substances and the temperature. No matter what reaction or change in properties occurs, the total weight of the substances does not change. (Boundary: Mass and weight are not distinguished at this grade level.) By the end of grade 8. Substances react chemically in characteristic ways. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants. The total number of each type of atom is conserved, and thus the mass does not change. Some chemical reactions release energy, others store energy. Science for All Americans: THE EARTH The earth has a variety of climatic patterns, which consist of different conditions of temperature, precipitation, humidity, wind, air pressure, and other atmospheric phenomena. These patterns result from an interplay of many factors. The basic energy source is the heating of land, ocean, and air by solar radiation. Transfer of heat energy at the interfaces of the atmosphere with the land and oceans produces layers at different temperatures in both the air and the oceans. These layers rise or sink or mix, giving rise to winds and ocean currents that carry heat energy between warm and cool regions. The earth's rotation curves the flow of winds and ocean currents, which are further deflected by the shape of the land. The cycling of water in and out of the atmosphere plays an important part in determining climatic patterns—evaporating from the surface, rising and cooling, condensing into clouds and then into snow or rain, and falling again to the surface, where it collects in rivers, lakes, and porous layers of rock. There are also large areas on the earth's surface covered by thick ice (such as Antarctica), which interacts with the atmosphere and oceans in affecting worldwide variations in climate. STRUCTURE OF MATTER The things of the physical world seem to be made up of a stunningly varied array of materials. Materials differ greatly in shape, density, flexibility, texture, toughness, and color; in their ability to give off, absorb, bend, or reflect light; in what form they take at different temperatures; in their responses to each other; and in hundreds of other ways. Yet, in spite of appearances, everything is really made up of a relatively few kinds of basic material combined in various ways. As it turns out, about 100 such materials—the chemical elements—are now known to exist, and only a few of them are abundant in the universe. When two or more substances interact to form new substances (as in burning, digestion, corrosion, and cooking), the elements composing them combine in new ways. In such recombinations, the properties of the new combinations may be very different from those of the old. An especially important kind of reaction between substances involves combination of oxygen with something else—as in burning or rusting. The basic premise of the modern theory of matter is that the elements consist of a few different kinds of atoms—particles far too tiny to see in a microscope—that join together in different configurations to form substances. There are one or more—but never many—kinds of these atoms for each of the approximately 100 elements. There are distinct patterns of properties among the elements. There are groups of elements that have similar properties, including highly reactive metals, less-reactive metals, highly reactive non-metals (such as chlorine, fluorine, and oxygen), and some almost completely nonreactive gases (such as helium and neon). Some elements don't fit into any of these categories; among them are carbon and hydrogen, essential elements of living matter. When the elements are listed in order by the masses of their atoms, similar sequences of properties appear over and over again in the list. Benchmarks for Science Literacy: THE EARTH By the end of the 2nd grade, students should know that The temperature and amount of rain (or snow) tend to be high, low, or medium in the same months every year. 4B/P1* Water can be a liquid or a solid and can go back and forth from one form to the other. If water is turned into ice and then the ice is allowed to melt, the amount of water is the same as it was before freezing. 4B/P2 Water left in an open container disappears, but water in a closed container does not disappear. 4B/P3 By the end of the 5th grade, students should know that When liquid water disappears, it turns into a gas (vapor) in the air and can reappear as a liquid when cooled, or as a solid if cooled below the freezing point of water. Clouds and fog are made of tiny droplets or frozen crystals of water. 4B/E3* MATTER K-2 Students should examine and use a wide variety of objects, categorizing them according to their various observable properties. They should subject materials to such treatments as mixing, heating, freezing, cutting, wetting, dissolving, bending, and exposing to light to see how they change. Even though it is too early to expect precise reports or even consistent results from the students, they should be encouraged to describe what they did and how materials responded. Students should also get a lot of experience in constructing things from a few kinds of small parts ("Tinkertoys" and "Legos"), then taking them apart and rearranging them. They should begin to consider how the properties of objects may differ from properties of the materials they are made of. And they should begin to inspect things with a magnifying glass to discover features not visible without it. By the end of the 2nd grade, students should know that Objects can be described in terms of their properties. Some properties, such as hardness and flexibility, depend upon what material the object is made of, and some properties, such as size and shape, do not. 4D/P1* Things can be done to materials to change some of their properties, but not all materials respond the same way to what is done to them. 4D/P2 3-5 The study of materials should continue and become more systematic and quantitative. Students should design and build objects that require different properties of materials. They should write clear descriptions of their designs and experiments, present their findings whenever possible in tables and graphs (designed by the students, not the teacher), and enter their data and results in a computer database. Objects and materials can be described by more sophisticated properties—conduction of heat and electricity, buoyancy, response to magnets, solubility, and transparency. Students should measure, estimate, and calculate sizes, capacities, and weights. If young children can't feel the weight of something, they may believe it to have no weight at all. Many experiences of weighing (if possible on increasingly sensitive balances)—including weighing piles of small things and dividing to find the weight of each—will help. It is not obvious to elementary students that wholes weigh the same as the sum of their parts. That idea is preliminary to, but far short of, the conservation principle to be learned later that weight doesn't change in spite of striking changes in other properties as long as all the parts (including invisible gases) are accounted for. With magnifiers, students should inspect substances composed of large collections of particles, such as salt and talcum powder, to discover the unexpected details at smaller scales. They should also observe and describe the behavior of large collections of pieces—powders, marbles, sugar cubes, or wooden blocks (which can, for example, be "poured" out of a container) and consider that the collections may have new properties that the pieces do not. By the end of the 5th grade, students should know that Heating and cooling can cause changes in the properties of materials, but not all materials respond the same way to being heated and cooled. 4D/E1a* Many kinds of changes occur faster under hotter conditions. 4D/E1b No matter how parts of an object are assembled, the weight of the whole object is always the same as the sum of the parts; and when an object is broken into parts, the parts have the same total weight as the original object. 4D/E2* Materials may be composed of parts that are too small to be seen without magnification. 4D/E3 When a new material is made by combining two or more materials, it has properties that are different from the original materials. 4D/E4a A lot of different materials can be made from a small number of basic kinds of materials. 4D/E4b* Substances may move from place to place, but they never appear out of nowhere and never just disappear. 4D/E5** (ASL) All materials have certain physical properties, such as strength, hardness, flexibility, durability, resistance to water and fire, and ease of conducting heat. 4D/E6** (SFAA) Collections of pieces (powders, marbles, sugar cubes, or wooden blocks) may have properties that the individual pieces do not. 4D/E7** (ASL) PLANNING RESOURCES Big Ideas: The Universe consists of matter and energy, which is continually being changed and transferred throughout the Earth and Universe. The Sun is the major source of energy for the Earth. Energy from the Sun drives the water cycle. Essential Questions: Why is the Sun important? How does the Sun impact the Earth? What is the water cycle? Why is the water cycle an important process for Earth? How is the Sun connected to the water cycle? What is matter? What is mass? What is weight? How are mass and weight related? How can we describe matter? How can matter be changed? How can we describe the changes that take place in matter? Enduring Understandings: No matter how parts of an object are assembled, the weight of the whole object is always the same as the sum of the parts; and when an object is broken into parts, the parts have the same total weight as the original object. Matter has many properties and can be changed. Changes in matter can be described in terms of physical and chemical properties. When a new material is made by combining two or more materials, it has properties that are different from the original materials. Identify Misconceptions: Use formative probes: Uncovering Student ideas in Science, Volumes 1-4, by Page Keeley (I) Volume1 Ice Cubes in a Bag p. 49 (II) Volume 1 Lemonade p. 55 (III) Volume 1 Cookie Crumbles, p. 61 (IV) Volume 1 Seedlings in a Jar p. 67 (V) Volume 1 Is it Melting? P. 73 (VI) Volume 1 Is it Matter? P. 79 (VII) Volume 1 Is it Made of Molecules? P. 85 (VIII) Volume 1 The Rusty Nails p. 91 (IX) Volume 1 Wet jeans p. 155 (X) Volume 4 Sugar Water p. 11 (XI) Volume 4 Burning Paper p. 23 (XII) Volume 4 Nails in a Jar p. 31 (XIII) Volume 4Standing on One Foot p. 61 Formative Assessment Probes (articles, how-to, free-online) by Page Keeley, et al http://pal.lternet.edu/docs/outreach/educators/education_pedagogy_research/assessment_probes_unc overing_student_ideas.pdf http://www.ode.state.or.us/teachlearn/subjects/science/resources/msef2010formative_assessment_probes.pdf Annotated TEACHING Resources: Adventures in Chemistry http://www.acs.org/content/acs/en/education/whatischemistry/adventures-in-chemistry.html An assortment of chemistry activities form the American Chemical Society. NOAA Water Cycle Game http://prisms.mmsa.org/review.php?rid=1308 The resource is a role-playing game in which students take on the role of a water molecule and travel through nine compartments of the water cycle to gain a better understanding for the true complexity of the movement of water. The Water Cycle http://prisms.mmsa.org/review.php?rid=1291 The representation features a detailed six minute animated lesson about the major processes that move water between land, the ocean and the atmosphere, and convert water between states. Evaporation, condensation, transpiration and water reservoirs are major topics covered by the animation. Thirstin's Water Cycle: Rain http://prisms.mmsa.org/review.php?rid=1319 The representation is an animation of the water cycle. The user can select individual parts, such as: rain, water vapor, water storage and clouds. The user can observe water as it cycles through the various parts of the water cycle. This review focuses on "R for Rain." The Hydrologic Cycle http://prisms.mmsa.org/review.php?rid=1283 The representation is an animation depicting the water cycle. The first page is an introduction with links to pages on condensation, precipitation, infiltration, runoff and evaporation and transpiration. A Summary of the Hydrologic Cycle http://prisms.mmsa.org/review.php?rid=1301 The representation is an animation of the water cycle, accompanied by a descriptive text that includes the topics of evaporation, condensation, precipitation, and transport using an additional diagram. NOAA Water Cycle Game http://prisms.mmsa.org/review.php?rid=1307 The resource is a role-playing game in which students take on the role of a water molecule and travel through nine compartments of the water cycle to gain a better understanding for the true complexity of the movement of water What are Physical Properties and Changes? http://www.elmhurst.edu/~chm/vchembook/104Aphysprop.html This short reference article describes, defines and lists several physical properties and physical changes. Photographs are included. Water Properties http://ga.water.usgs.gov/edu/waterproperties.html Simple description of the chemical and physical properties of water produced by the U.S. Geological Survey. Investigating Changes of State: Chemical and Physical Changes http://serc.carleton.edu/sp/mnstep/activities/20101.html In this activity students explore and identify chemical and physical changes by observing a variety of changes in matter in lab stations and through the making of butter and pancakes. Physical and Chemical Changes http://ia.usu.edu/viewproject.php?project=ia:15350 Physical and chemical changes in matter affect us every day. Use the following resources to help you understand these changes more completely. Chemistry: classifying chemical and physical changes in various materials/substances http://serc.carleton.edu/sp/mnstep/activities/26440.html This activity is a classroom lab where students observe and classify chemical and physical changes using the five characteristics of a chemical change, interpret their findings, and use evidence to support their findings. Forgotten Genius http://www.pbs.org/wgbh/nova/julian/program.html This is a 2 hour program divided into 13 chapters, describing the life and accomplishments of Percy Julian. http://www.pbs.org/wgbh/nova/teachers/activities/pdf/3402_julian.pdf This series of chemistry stations are designed to accompany the PBS documentary about AfricanAmerican chemist "Percy Julian: Forgotten Genius." Each of the six stations features either a chemical or physical change: mixing vinegar and metals (chemical), baking soda and vinegar (chemical), antacid tablets and water (chemical), cabbage juice indicator and... The Magic of Matter Discover what matter is, the different kinds of matter, and how it changes. Your assignment - have fun with matter! http://ia.usu.edu/viewproject.php?project=ia:7086 Mystery Mud: Exploring Changes in States of Matter http://www.teachersdomain.org/resource/phy03.sci.phys.matter.mud/Join a group of middle-school students on a visit to a laboratory at the Massachusetts Institute of Technology, where they experiment with "mystery mud" and learn about the relationships between magnetism, particle motion, and changes in the state of matter. Barfing Pumpkin http://www.teachersdomain.org/resource/odc08.scitech.matter-energy.barfingpumpkin/ This demonstration shows a chemical change with hydrogen peroxide decomposing into water and oxygen. Video Resources: Bill Nye Phases of Matter (1,2,&3) http://www.gamequarium.org/cgi-bin/search/linfo.cgi?id=7685 Bill Nye Chemical Reactions (1,2, &3) http://www.gamequarium.org/cgi-bin/search/linfo.cgi?id=7907 Teachers Domain Free digital media for educational use. http://www.teachersdomain.org/ Essential Science for Teachers: Physical Science http://www.learner.org/resources/series200.html Physical Science consists of eight one-hour video programs accompanied by print and Web materials that provide in-class activities and homework explorations. Real-world examples, demonstrations, animations, still graphics, and interviews with scientists compose content segments that are intertwined with indepth interviews with children that uncover their ideas about the topic at hand. Each program also features an elementary school teacher and his or her students exploring the topic using exemplary science curricula. Use the complete course for teacher education or professional development, or individual programs for content review. Text Resources: http://www.chem4kids.com/ http://www.sciencekids.co.nz/chemistry.html http://www.ducksters.com/science/chemistry/ https://kids.usa.gov/teens/science/chemistry/index.shtml Terminology: evaporation transpiration condensation precipitation runoff matter energy Writing Prompts: 1. Many people have favorite foods that they snack on. Do you have a favorite snack food? Describe your favorite snack food. Explain what you think is in your snack food that makes it so appealing to you. 2. Imagine you are a drop of water falling from the sky as rain. Describe your adventure as you land on the earth, move towards the ocean, and ultimately are evaporated or transpired back into the atmosphere. 3. You have decided to bake a batch of cupcakes to share with your friends at school. Describe the process you will go through to prepare the cupcakes for your classmates. 4. After burning for 3 hours, a candle has lost half of its mass. Write an essay explaining where the mass has gone. 5. Your mom has asked you to clean the sliding glass doors that lead out to your play area in the yard. However, there is no window cleaner left in the bottle. Your mom tells you that you can mix water with white vinegar or the juice from a lemon to make some home-made window cleaner. Which of these household chemicals would you use, and why?