Bohr Atom In 1911, Rutherford introduced a new model of the atom
advertisement

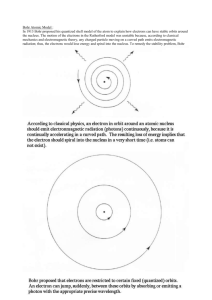
Bohr Atom In 1911, Rutherford introduced a new model of the atom in which cloud of negatively charged electrons surrounding a small, dense, positively charged nucleus. This model is result of experimental data and Rutherford naturally considered a planetary-model atom. The laws of classical mechanics (i.e. the Larmor formula, power radiated by a charged particle as it accelerates.), predict that the electron will release electromagnetic radiation while orbiting a nucleus. Because the electron would lose energy, it would gradually spiral inwards, collapsing into the nucleus. This atom model is disastrous, because it predicts that all atoms are unstable. To overcome this difficulty, Niels Bohr proposed, in 1913, what is now called the Bohr model of the atom. He suggested that electrons could only have certain classical motions: 1. The electrons can only travel in special orbits: at a certain discrete set of distances from the nucleus with specific energies. 2. The electrons of an atom revolve around the nucleus in orbits. These orbits are associated with definite energies and are also called energy shells or energy levels. Thus, the electrons do not continuously lose energy as they travel in a particular orbit. They can only gain and lose energy by jumping from one allowed orbit to another, absorbing or emitting electromagnetic radiation with a frequency ν determined by the energy difference of the levels according to the Planck relation: Δ𝐸 = 𝐸2 − 𝐸1 = ℎ𝜈 3. Kinetic energy of the electron in the orbit is related to the frequency of the motion of the electron: 1 1 𝑚𝑣 2 = 𝑛ℎ𝜈 2 2 For a circular orbit the angular momentum L is restricted to be an integer multiple of a fixed unit: 𝐿 = 𝑚𝑣𝑟 = 𝑛ℏ where n = 1, 2, 3, ... is called the principal quantum number. The lowest value of n is 1; this gives a smallest possible orbital radius of 0.0529 nm known as the Bohr radius. Bohr's condition, that the angular momentum is an integer multiple of ħ was later reinterpreted by de Broglie as a standing wave condition: the electron is described by a wave and a whole number of wavelengths must fit along the circumference of the electron's orbit: 𝑛𝜆 = 2𝜋𝑟 The Bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. To calculate the orbits requires two assumptions: 1. (Classical Rule)The electron is held in a circular orbit by electrostatic attraction. The centripetal force is equal to the Coulomb force. 𝑚𝑣 2 𝑍𝑒 2 = 𝑟 4𝜋𝜖0 𝑟 2 It also determines the total energy at any radius: 1 𝑍𝑒 2 𝑍𝑒 2 𝐸 = 𝑚𝑣 2 − =− 2 4𝜋𝜖0 𝑟 8𝜋𝜖0 𝑟 The total energy is negative and inversely proportional to r. This means that it takes energy to pull the orbiting electron away from the proton. For infinite values of r, the energy is zero, corresponding to a motionless electron infinitely far from the proton. 2. (Quantum rule) The angular momentum 𝐿 = 𝑚𝑣𝑟 = 𝑛ℏ, so that the allowed orbit radius at any n is: 𝑛 2 ℏ2 𝑟𝑛 = 4𝜋𝜖0 2 𝑍𝑒 𝑚 The energy of the n-th level is determined by the radius: 2 𝑍𝑒 2 𝑍𝑒 2 𝑚 𝑍 2 13.6 𝐸=− = −( ) = − 𝑒𝑉 8𝜋𝜖0 𝑟𝑛 4𝜋𝜖0 2ℏ2 𝑛2 𝑛2 An electron in the lowest energy level of hydrogen (n = 1) therefore has 13.6 eV less energy than a motionless electron infinitely far from the nucleus. The combination of natural constants in the energy formula is called the Rydberg energy (RE): 2 𝑒2 𝑚 𝑅𝐸 = ( ) 4𝜋𝜖0 2ℏ2 This expression is clarified by interpreting it in combinations which form more natural units. We define 𝑚𝑐 2 is rest mass energy of the electron (511 keV) and 𝑒2 4𝜋𝜖0 ℏ𝑐 = 𝛼 is the fine structure constant then 𝑅𝐸 = 1 (𝑚𝑐 2 )𝛼 2 2 Bohr Atom and Rydberg formula The Rydberg formula, which was known empirically before Bohr's formula, is now in Bohr's theory seen as describing the energies of transitions or quantum jumps between one orbital energy level, and another. When the electron moves from one energy level to another, a photon is emitted. Using the derived formula for the different 'energy' levels of hydrogen one may determine the 'wavelengths' of light that a hydrogen atom can emit. The energy of a photon emitted by a hydrogen atom is given by the difference of two hydrogen energy levels: 𝐸 = 𝐸𝑖 − 𝐸𝑓 = 𝑅𝐸 ( 1 1 2 − 2) 𝑛𝑓 𝑛𝑖 where nf is the final energy level, and ni is the initial energy level. Since the energy of a photon is 𝐸 = ℎ𝑐 𝜆 , the wavelength of the photon given off is given by 1 1 1 = 𝑅( 2 − 2 ) 𝜆 𝑛𝑓 𝑛𝑖 This is known as the Rydberg formula, and the Rydberg constant R is RE / hc. This formula was known in the nineteenth century to scientists studying spectroscopy, but there was no theoretical explanation for this form or a theoretical prediction for the value of R, until Bohr. In fact, Bohr's derivation of the Rydberg constant, as well as the concomitant agreement of Bohr's formula with experimentally observed spectral lines of the Lyman (nf = 1), Balmer (nf = 2), and Paschen (nf = 3) series, and successful theoretical prediction of other lines not yet observed, was one reason that his model was immediately accepted. Improvement of Bohr Model Several enhancements to the Bohr model were proposed; most notably the Sommerfeld model or Bohr-Sommerfeld model, which suggested that electrons travel in elliptical orbits around a nucleus instead of the Bohr model's circular orbits. This model supplemented the quantized angular momentum condition of the Bohr model with an additional radial quantization condition, the Sommerfeld-Wilson quantization condition 𝑇 ∫ 𝑝𝑟 𝑑𝑞𝑟 = 𝑛ℎ 0 where pr is the radial momentum canonically conjugate to the coordinate q which is the radial position and T is one full orbital period. The Bohr-Sommerfeld model was fundamentally inconsistent and led to many paradoxes. The Sommerfeld quantization can be performed in different canonical coordinates, and sometimes gives answers which are different. In the end, the model was replaced by the modern quantum mechanical treatment of the hydrogen atom, which was first given by Wolfgang Pauli in 1925, using Heisenberg's matrix mechanics. The current picture of the hydrogen atom is based on the atomic orbitals of wave mechanics which Erwin Schrödinger developed in 1926. However, this is not to say that the Bohr model was without its successes. Calculations based on the Bohr-Sommerfeld model were able to accurately explain a number of more complex atomic spectral effects. References http://en.wikipedia.org/wiki/Bohr_model