Ionization energy
advertisement

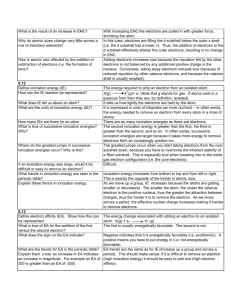
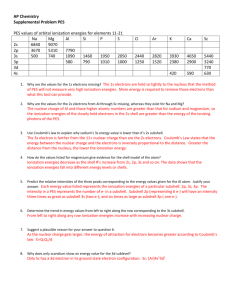
Accelerated Chemistry – Unit 11 Worksheet 1 Ionization energy Introduction: Ionization Energy is the energy needed to remove the outermost electron from an atom. 1) Given your knowledge of the nucleus of an atom and electrons (specifically their charges), why would energy be needed to remove the outermost electron from an atom? 2) If an electron is removed from an atom, would the atom be neutral or have a charge? Does this explain why it is called ionization energy? Explain. Proceed to http://pieperphs.pbworks.com Open the Ionization Energy and Atomic Radius link. There are two pages to this Excel document; Make sure you are on the IE page, check at the bottom. Examine the chart of ionization energies of elements and their atomic number (Z) related to the removal of each successive electron (1st electron removed from the atom, 2nd electron removed from the atom, etc.) These values represent the energy needed to remove an electron one at a time from an atom. This is why Li, for example, has three values because after removing the 3rd electron, it has no electrons left. Plot graphs of successive ionization energies for all electrons for B, Si, and Ca. Highlight the 5 cells below the 5 (atomic number) of B. These numbers represent the energy values needed to remove 5 electrons (one at a time) from Boron. Click on the Insert link at the top of the spreadsheet Click on Scatter at the top of the spreadsheet and click on the graph with no lines (upper left) Click on Layout at the top and then add Chart Title(Boron) to your graph Also click on Axis Title and add these to your graph (electrons on x-axis and IE on y-axis) Repeat the same graphing process with all 14 cells for Silicon (atomic number, 14) and with all 20 cells for Calcium (atomic number, 20). Display all three graphs at the bottom of the spreadsheet. ©Modeling Instruction – AMTA 2012 1 U11 ws1 v1 3) Compare the graphs – what are the common features? What are the differences? 4) Group the electrons for each element based on ionization energies. How many electrons are in each group? You can change the scale of the vertical axis to better differentiate between groups. (Specifically, RIGHT Click on the y-axis values for the Silicon graph, select Format Axis then select Fixed for the maximum amount and type in 600. Although you lose two points (electrons) in the process, it helps to see the separation points with the remaining points (electrons). I would do the same with the y-axis values for Calcium but do it once with the setting if 1200 and a second time with 500. Again this will help you see the groupings of points (electrons). 5) Is there a maximum number of electrons that can fit in each group? If so, what is it? 6) Plot graphs of successive ionization energies for three more atoms. Which atoms did you plot and does the evidence from these plots support your answer to #5? 7) Summarize the patterns you found in the table of successive ionization energies. ©Modeling Instruction – AMTA 2012 2 U11 ws1 v1 Extension: 1. Draw Bohr model diagrams for Boron, Nitrogen, Fluorine and Sodium; Use these diagrams to explain the trend in the first ionization energy as you move across a period. Explain why IE1 for Sodium is so much lower than it is for Fluorine. 2. Now use these diagrams to explain the trend in atomic radii as you move across a period. Explain why Fluorine is smaller than Oxygen, yet Sodium is not smaller than Fluorine. 3. Using the diagrams you drew and your graphs, what is the trend in first ionization energy for atoms going down a group on the periodic table? What is the trend for atomic radius going down a group? ©Modeling Instruction – AMTA 2012 3 U11 ws1 v1