The Mole - West Linn High School
advertisement

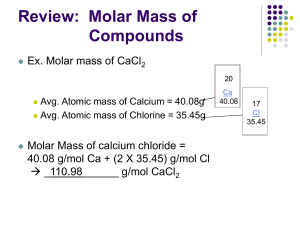
Chemistry 1 West Linn High School Unit 4 Packet and Goals Name:_________________________________ Period:_________ Unit 4 – Formulas, Names, and Quantities of Compounds Unit Goals: As you work through this unit, you should be able to: 1. Distinguish between Ionic and Molecular Compounds. (7.2, 8.1) 2. Distinguish between chemical formulas, molecular formulas, and formula units. (9.1-9.3) 3. Know the charges and the formulas for monatomic ions using the periodic table and common polyatomic ions. (9.1) 4. Apply the rules for naming and writing formulas for binary and ternary ionic compounds. (9.2) 5. Apply the rules for naming and writing formulas for binary molecular compounds. (9.3) 6. Define a mole in chemistry. (10.1) 7. Describe the 3 quantities a mole in equal to. (10.1) 8. Use moles to convert among measurements of mass, number of particles, and volume. (10.2) 9. Calculate the percent composition of a substance from its chemical formula or experimental data. (10.3) 10. Derive the empirical formula and molecular formula of a compound from experimental data. (10.3) Reading: Chapter 7: (pp 201-207) Section 7.2 Chapter 8: (pp 222-225) Section 8.1 Chapter 9: (pp 262-283) Sections 9.1-9.3 Chapter 10: (pp 304-341) Sections 10.1 – 10.3 Key Terms: ionic bond, ionic compound, covalent bond, molecular compound, chemical formula, formula unit, molecular formula, monatomic ion, polyatomic ion, binary compound, ternary compound, mole, Avogadro’s number, molar mass, molar volume, STP (standard temperature and pressure), percent composition, molecular formula, empirical formula Classwork and Labs Homework: HW 1 Description Ionic vs Molecular Compounds WS Goals 1-2 Score Unit 4 Note Packet Naming Activity HW 2 HW 3 HW 4 Writing the names and formulas of Ionic Compounds WS 3-4 Writing the names and formulas of Molecular Compounds WS 5 Mole Conversions WS 6-8 Ionic Naming Quiz Naming Mastery Quiz Means of counting Activity Mole Conversions Mastery Quiz % Composition Lab Unit 5 Exam/Semester Final Exam HW 5 Percent Composition and Empirical Formula WS 9-10 Empirical Formula Lab (Start of 2nd Semester) Chapters 7 and 8: Ionic vs. Molecular Compounds 7.2 Ionic Bonds and Ionic Compounds Formation of Ionic Compounds: Page 201 Define Ionic Compound: A compound composed of cations (+ charged, usually a metal) & anions (- charged, usually a non-metal) Define Ionic Bond: The electrostatic attraction between the + cations & - anions in an ionic compound. Electrons are totally lost or gained. There is no sharing of electrons in ionic bonds. 8.1. Molecular Compounds Molecules and Molecular Compounds: (page 222) Covalent Bond The attraction created when non-metallic atoms share electrons. Molecule A group of atoms joined by the sharing of electrons. Usually occurs between non-metallic atoms. Comparing Molecular and Ionic Compounds: Page 224 Ionic Compounds Strong attractive forces between metals & non-metals Always results in a solid substance Repeating ion-ion pattern called a crystal lattice High melting and boiling points Cations & anions Molecular Compounds Weak attractive forces Usually forms liquids & solids Usually all non-metallic atoms Low melting & boiling points Chapter 9 Naming Ionic & Molecular Compounds Chemical Formulas Formula unit Molecular formula The lowest whole # ratio of ions in an ionic compound The chemical formula for a covalent Similar to a molecule, but this refers to ionic substances compound that shows the kinds and #’s of atoms in a molecule of a compound 9.1 Naming Ions Monoatomic Ions: (page 264) • Consist of a single ion with a positive or negative charge Na+ Ca2+ Representative Elements: KNOW THE CHARGES! All groups IA – 8A – Cations: Name is the name of the element » Ex) Ca2+ = Calcium ion – Anions: Name ending changed to end in “ide” » Ex) O2- = Oxide Transition Metals: CAN HAVE MULTIPLE CHARGES! All metals with electrons in d-orbitals – Cations: Name must give charge (Use Roman Numeral) » Ex) Cu2+ = Copper (II) Cu1+ = Copper (I) Sample Problem 9.1 Name the ion formed by each of the following elements. a. potassium b. lead, 4 electrons lost potassium cation lead (IV) cation Example Problems 1. Name the ions formed by the following elements. a. selenium Selenide anion b. barium barium cation c. phosphorus phosphide anion d. iodine iodide anion c. sulfur sulfide anion 2. How many electrons were lost or gained? a. Iron (III) 3 lost b. Oxide 2 gained c. Copper (I) 1 lost d. Strontium ion 2 lost Polyatomic Ions: (page 268) • Composed of more than one atom • Molecules with Charge • Need to memorize or have access to list. • Page 268 • Items to Know Sheet for Naming Nitrate, sulfate, phosphate, hydroxide, carbonate, etc.. 9.2 Naming and Writing Formulas for Ionic Compounds Binary Ionic Compounds: (page 271) An ionic compound that is composed of only two different elements. NaCl, Ca3P2, K2O Ionic Compound Example • Do the following compounds exist? – LiO? No, it would result in a -1 charged particle – Li2O? Yes, it forms a neutral particle – LiO2? No, it would result in a -3 charged particle – Li2O2? No, it would result in a -2 charged particle Ionic Compound Naming Rules – Cation is always named first – Name the cation (usually the metal ion) using the same name as the element – If the cation is a transition metal, use a Roman numeral following the element name to indicate the charge Be Careful: Only Cations that form multiple ions! – Name the anion If a single element, replace the last syllable with “ide” If a polyatomic ion, just use its namePractice: Name the following • LiBr lithium bromide • CaO calcium oxide • Sr(NO2)2 strontium nitrite • Rb2SO4 rubidium sulfate • Fe2O3 iron(III) oxide • CaCr2O7 calcium dichromate • Na2CO3 sodium carbonate Formula Rules: • The Formula must be “Balanced” with lowest whole # ratio of ions • The number of each ion depends on its charge and the charge of the other ion. • Try Crossing the Charges Practice: Write the formula for the following ionic compounds • Sodium oxide Na2O • Copper (I) sulfide Cu2S • Barium oxide BaO • Copper (II) sulfate CuSO4 • Aluminum oxide Al2O3 • Ammonium phosphate (NH4)3PO4 • Calcium chloride CaCl2 • Iron (III) nitrate Fe(NO3)3 9.3 Naming and Writing Formulas for Molecular Compounds Binary Molecular Compounds (Page 280) 1. Write the names of the elements in the order listed in the formula 2. Use prefixes to indicate the number of each kind of atom. Exeption: If the first element is 1 don’t use mono 1. End the name of the second element with the suffix “ide” Practice: Name the Following Compounds • CO carbon monoxide • CO2 carbon dioxide • N2O4 dinitrogen tetraoxide • PCl5 phosphorus pentachloride • XeF6 xenon hexafluoride • CCl4 carbon tetrachloride Prefix mono di tri tetra penta hexa hepta octa nona deca Number 1 2 3 4 5 6 7 8 9 10 Chapter 10 10.1. The Mole: A Measurement of Matter What is a Mole: Page 308 What is a mole? • A mole is similar to the concept of a dozen – Why do you count cookies in dozens? It’s a fast way to categorize or group large amounts of items – Why do you count chemistry particles in moles? It’s an easy way to group or categorize extremely large quantities of atoms or particles • • How big is Avogadro's number? – An Avogadro's number of soft drink cans would cover the surface of the earth to a depth of over 200 miles. – If you spread Avogadro's number of unpopped popcorn kernels across the USA, the entire country would be covered in popcorn to a depth of over 9 miles. – If we were able to count atoms at the rate of 10 million per second, it would take about 2 billion years to count the atoms in one mole. – If you count out loud starting with the number "one" at the rate of one count every second, it may take you about 1,909,577,942,668,696 years to finish. This is roughly 960,000 times the estimated lifetime of our universe (assuming 20 Billion years). – Using a Pentium 450 MHz CPU, it will still take about 4,243,506 years to finish this task. This is a period of time about a thousand times longer than the total span of our civilization. – If marbles that have a diameter of one centimeter were to be lined up end-to-end in a straight line, the distance covered by this string of marbles can hold in about 500,000,000 of our Solar System placed end-to-end. What is a Particle? It depends on the substance and situation – Atoms, Molecules, Formula Units are all particles – Element: ___atom__________________________________ – Diatomic Element: _ molecule_________________________________ – Molecular Compound: _ molecule _____________________________ – Ionic Compound: _ formula unit________________________________ Mole Conversions # Particles x Moles x 1 mole 6.02x1023 particles 6.02x1023 particles 1 mole = ________ moles = ________ Particles Example Problems 1) 3.2x1024 molecules of water are how many moles of water molecules? 2) 1.50 mol of Carbon dioxide is how many molecules? 3) 0.10 mol of NaCl is how many formula units? Sample Problem 10.2 (page 309) Magnesium is a light metal used in the manufacture of aircraft, automobile wheels, and tools. How many moles of magnesium are 1.25 x 1025 atoms of magnesium? Sample Problem 10.2 (page 311) Propane is a gas used for cooking and heating. How many atoms are in 2.12 mol of propane (C 3H8)? Molar Mass: Page 312 Why is a mole 6.02 x 1023? • Mole: 6.02 x 1023 particles Molar Mass = The mass of 6.022 x 1023 particles Mole Examples Substance Representative Particle # of Atoms or Ions Mass of 1 Particle Mass of 1 Mole NaCl Formula units 2 58amu 58g Copper Cu Atom 1 64amu 64g Water H2O Molecule 3 18amu 18g Hydrogen gas H2 Molecule 2 2amu 2g Sample Problem 10.4 (page 315) The decomposition of hydrogen peroxide (H2O2) provides sufficient energy to launch a rocket. What is the molar mass of hydrogen peroxide? There are 2 moles of hydrogen and 2 moles of oxygen in a mole of hydrogen peroxide. Each mole of hydrogen has a mass of 1g and each mole oxygen has a mass of 16g. The total mass is 34g per mole. Additional Examples: What are the molar masses of the following compounds? Ag2SO4 Mg(NO3)2 Ag = 108*2 = 216 Mg = 24 S = 32 N = 14*2 = 28 O = 16*4 = 64 O = 16*6 = 96 Total = 312g Total = 148g 10.2. Mole – Mass & Mole – Volume Relationships The Mole – Mass Relationship: Page 201 Mass x Moles x 1 mole (Molar mass) (Molar Mass) 1 mole = _______ moles = ________ mass Example Problems 1) 23.8 g of water contains how many moles of water molecules? 2) 1.50 mol of Carbon dioxide has what mass? 3) 0.10 mol of NaCl has what mass? C6H12O6 C = 12*6 = 72 H = 1*12 = 12 O = 16*6 = 96 Total = 180g Sample Problem 10.5 (page 318) What is the mass, in grams, of 9.45 mol of aluminum oxide? Sample Problem 10.6 (page 319) How many moles of iron(III) oxide are contained in 92.2 g of pure Fe2O3? The Mole – Volume Relationship: Page 320 • For gasses, a mole is also related to Volume • At a common temperature and pressure (STP) • 1 mole = 22.4 L • STP = Standard Temperature and Pressure • STP is 0⁰C and 1 atm of pressure (sea level on earth) At STP Volume x Moles x 1 mole 22.4 L 22.4 L 1 mole = ________ moles = ________ mass Example Problems 1) 34.6 L of Oxygen at STP is how many moles of Oxygen? 2) 1.50 mol of Carbon dioxide is what volume at STP? 3) 0.10 mol of N2 is what volume at STP? Sample Problem 10.7 (page 321) Determine the volume, in liters, of 0.60 mol SO2 gas at STP. Calculating Molar Mass and Density: Page 322 The Density of any gas can be found at STP because: D = Mass/Volume 1 mole of gas at STP = Molar Mass 1 mole of gas at STP = 22.4 L What is the Density of O2 at STP? Sample Problem 10.8 (page 322) The Density of a gaseous compound containing carbon and oxygen is found to be 1.964 g/L at STP. What is the molar mass of the compound? Mole Roadmap: Page 323 • 1 mole is: • 6.02x1023 particles • Molar mass of substance • 22.4 L of Volume at STP for ALL gases Molar Mass 1 Mole Factor Label Method of problem solving 1 mol = molar mass 1 mol = 6.02x1023 particles 1 mol = 22.4 L at STP Example Problems: 1. Mass of 2.5x1024 molecules of O2 gas? 2. Number of molecules in 26.5 g of CO2? 3. Mass of 1.5 x 1018 atoms of Helium? 4. Mass of 42.2 L of Helium gas at STP? 22.4 L of gas at STP 6.02x1023 Particles 7.3 Percent Composition and Chemical Formulas Percent Composition of a Compound: (page 325) Math Warm up: 1. What is 73% of 150? 0.73 x 150 = 109.5 2. What percentage of 6.5 is 3.1? 6.5 x 0.031 = 0.202 Percent Composition: 1. Calculating % from Experimental Data – Based on MASS (not moles or # of particles) – For a compound it depends on the Formula 𝑚𝑎𝑠𝑠 𝑜𝑓 𝐸𝑙𝑒𝑚𝑒𝑛𝑡 % 𝑏𝑦 𝑚𝑎𝑠𝑠 = 𝑚𝑎𝑠𝑠 𝑜𝑓 𝐶𝑜𝑚𝑝𝑜𝑢𝑛𝑡 𝑋 100% When a 13.60 g sample of a compound containing only magnesium and oxygen is decomposed, 5.40 g of oxygen is obtained. What is the percent composition of this compound? 5.40g / 13.60g x 100 = 39.7% 2. Calculating % from Formulas and Molar Mass 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝑜𝑓 𝐸𝑙𝑒𝑚𝑒𝑛𝑡 𝑖𝑛 𝐶𝑜𝑚𝑝𝑜𝑢𝑛𝑑 % 𝑏𝑦 𝑚𝑎𝑠𝑠 = 𝑋 100% 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝑜𝑓 𝐶𝑜𝑚𝑝𝑜𝑢𝑛𝑡 What is the percent composition of the elements in Propane (C3H8)? C = 3 x 12g = 36g H = 8 x 1g = 8g C3H8 = 44g %C 36g / 44g = 0.818 or 81.8% %H 8g / 44g = 0.182 or 18.2% 3. Percent composition as a conversion factor How many grams of Carbon and Hydrogen are in 82.0 g of C3H8? 82.0g x 0.818 = 67.1g C 82.0g x 0.182 = 14.9 g H Empirical Formulas: page 330 • Lowest whole number ratio of elements in a compound • • Formula Units ARE Empirical Formulas Examples – NaCl – (NH4)2SO4 • • Molecular Formulas are not necessarily Empirical Formulas Examples – C2H6 – H2O2 – H2O Calculating Empirical Formulas 1. Find mass of each element in a compound 2. Convert masses to moles 3. Using the element with the smallest number of moles – Calculate the whole number “mole ratio” of each element (Divide each mole value by the smallest) Example What is the empirical formula for a compound which contains 41.1% iron, 23.6% sulfur and 35.3% oxygen? • First assume each % is a mass in g’s (corresponding to a total substance mass of 100.00 g). Find the atomic mass of each element from the periodic table and use it to convert the mass in grams to moles. • Next, convert the mole values to lowest whole number mole ratios. Do this by dividing each mole value by the smallest mole value of the group. These whole numbers tell the empirical formula ratios.