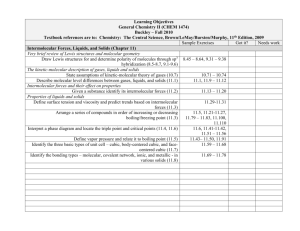
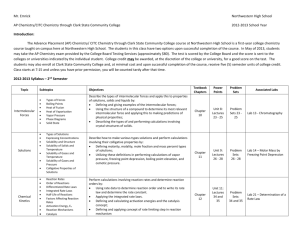
Unit Plan - Summary
advertisement
Cora Burt & Julie Wasylnka COURSE: Chemistry 12, University Preparation Unit of Study: Chemical systems and Equilibrium Course Code: SCH4U Summary Overall Expectations This unit focuses on the concept of systems in equilibrium and the application of these systems to chemical, ecological, biological, and industrial applications. The properties of these systems are examined and the factors that affect the extent of a chemical reaction are described. Experimentally, students will determine equilibrium constants and perform acidbase titrations. The knowledge of systems in equilibrium will allow students to better understand acid-base chemistry, as well as the solubility of compounds and their importance to qualitative analysis. A1. demonstrate scientific investigation skills (related to both inquiry and research) in the four areas of skills A2. identify and describe careers related to the fields of science under study, and describe the contributions of scientists, including Canadians, to those fields E1. analyse chemical equilibrium processes, and assess their impact on biological, biochemical, and technological systems; E2. investigate the qualitative and quantitative nature of chemical systems at equilibrium, and solve related problems; E3. demonstrate an understanding of the concept of dynamic equilibrium and the variables that cause shifts in the equilibrium of chemical systems. (1) Big Ideas “Chemical systems are dynamic and respond to changing conditions in predictable ways. Applications of chemical systems at equilibrium have significant implications for nature and industry.” (1) Key Questions What is chemical equilibrium? How do chemical reactions change in response to environmental stressors? How is chemical equilibrium important in day-to-day life? Unit of Study: Chemical systems and Equilibrium Assessment of Learning - Unit Test STSE Case Study: What’s in my Headache Medicine? Culminating Activity: Chemical Analyst for a Day Assessment for Learning - quizzes to assess understanding of conceptual theory Acid –Base Titrations in preparation for culminating activity Homework checks