Supplementary Table 4: Periprocedure and postoprocedure
advertisement

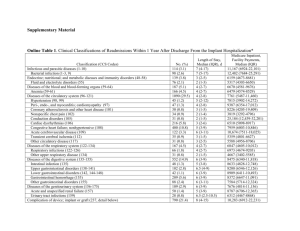
Supplementary Table 4: Periprocedure and postoprocedure complications with Endobarrier devices across different studies S.No Author/ Year/ Location Device/Int ervention Number of subjects Technique Duration of intervention Peri-procedure complication Minor adverse events (self limited/ mild -moderate) (procedure related) Major adverse events (severe)(procedure related) 1 Gersinn et al. 2007 U.S.A [5] Duodenojej unal bypass sleeve (DJBS) 1 DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system 12 weeks None None None 2 RodriguezGrunert et al. 2007 U.S.A. [6] 12 weeks Two (during explantation, no intervention required)a.) Oropharyngeal mucosal tear- 1/2 b.) Esophageal mucosal tear- 1/2 1. Abdominal pain- 6 2. Nausea- 18 3. Vomiting- 16 4. Diarrhoea- 1 5. Implant site inflammation- 12 Abdominal pain- 2- requiring early explantation of device (9 days after implant) (attributed to poor device placement) 1. Nausea- 10/21 2. Vomiting- 6/21 1. Upper abdominal pain14 2. Nausea- 6 3. Vomiting- 4 4. Constipation- 3 5. Dyspepsia- 2 6. Pyrexia- 2 7. Abdominal Pain- 2 1. Early device explantation (7/21)1.) Hematemesis- 3/21 at 11, 25 and 43 days post implant 2.) Abdominal pain/ nausea/vomiting- 4/21 at 3, 9, 30 and 36 days post implant None Not applicable (N/A) N/A 3 DJBS implant 12 DJBS implant Enrolled- 25 Successfully underwent implantation - 21 Gersin et al. 2009, U.S.A [7] Fluoroscope and Endoscope was used to deliver the DJBS implant over the wire catheter system DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system 12 weeks Esophagog astroduode noscopy (EGD) and mock implantatio n 26 Endoscopy S.No 4 5 Author/ Year/ Location Sandler et al. 2010, U.S.A [8] Rodrigruez et al. 2009, Chile [9] Device/Int ervention Gastroduod enojejunal Bypass Sleeve (GDJBS) Number of subjects Enrolled- 24 Successfully implanted22 Technique Endoscopically delivered with laparoscopic assistance DJBS implant 12 DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system EGD without implant 6 Endoscopy Duration of intervention 12 weeks Minor adverse events (self limited/ mild -moderate) (procedure related) Major adverse events (severe)(procedure related) None None 1. Pain with swallowing- 5/22, all 5 subjects underwent early explantation 2. Difficulty with transition to pureed diet- found to have kink within the stomach, endoscopy to correct the kink, no device explantation required. 1. Early device explantation(4/12)a.) Device migration/rotationi.) Symptomatic- 3/4 ii.) Asymtpomatic- 1/4 (discovered on scheduled endoscopy) N/A Peri-procedure complication 200 + 22 days 1. Nausea- 3/12 2. Vomiting- 2/12 1. Upper abdominal pain20 2. Nausea- 5 3. Vomiting- 7 4. Constipation- 1 5. Hypoglycemic symptoms5 6. Flatulence- 3 7. Abdominal Pain- 3 8. Diarrhoea- 1 9. Erosive duodenitis- 2 10. Gastritis- 1 11. Esophagitis- 1 190 + 44 days Data not available (DNA) N/A 2 Author/ Year/ Location Device/Int ervention Number of subjects Technique Duration of intervention Peri-procedure complication Minor adverse events (self limited/ mild -moderate) (procedure related) 6 Escalona et al. 2009, Chile [10] Flow restrictor DJBL implant (DJBL with a fluoropoly mer proximal plate with 4 mm orifice) Enrolled, who met study criteria- 10 DJBS implant was delivered with help of endoscope and fluoroscopy; 7/10 subjects required orifice dilatation (1/10- two times, 6/10- one time) 12 weeks 1. Nausea- 4/11 (36%) (ITT analysis) 2. Vomiting- 3/11 (27%) 1. Abdominal Pain- 7/11 (64%) (ITT analysis) 2. Nausea- 2/11 (18%) 3. Vomiting- 5/11 (46%) All of above symptoms improved with orifice dilation 7 Escalona et al. 2012, Chile [11] None 1. Upper abdominal pain81% 2. Nausea- 41% 3. Vomiting- 33% 4. Gastroenteritis- 4.8% S.No DJBS implant Enrolled- 42 Successfully implanted39 DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system 52 week 3 Major adverse events (severe)(procedure related) 1. Early device explantation(15/39)a.) Device migration/movement8/15 b.) Device obstruction- 3/15 c.) Abdominal pain- 2/15 d.) Acute cholecystitis- 1/15 e.) Patient request- 1/15 S.No 8 Author/ Year/ Location Munoz et al. 2013, Chile [12] Device/Int ervention DJBS implant Number of subjects 79 Technique DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system Duration of intervention 1 year 4 Peri-procedure complication DNA Minor adverse events (self limited/ mild -moderate) (procedure related) Major adverse events (severe)(procedure related) DNA 1. Early device explantation- 18/79 a.) Migration- 8/18 b.) Obstruction- 5/18 c.) Abdominal pain- 2/18 d.) Liver abscess- 1/18 e.) Upper GI bleed- 1/18 f.) Cholangitis- 1/18 g.) Ulcerative colitis- 1/18 h.) Acute cholecystitis- 1/18 i.) Patient request- 1/18 S.No 9 10 Author/ Year/ Location Schouton et al. 2010, Netherlands [13] Koehestanie et al. 2013, Netherlands [14] Device/Int ervention Number of subjects Technique DJBS implant + low calorie diet Enrolled- 30 Succesfully implanted26 DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system Low calorie diet only 11 N/A DJBS implant 28 DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system Duration of intervention Minor adverse events (self limited/ mild -moderate) (procedure related) Major adverse events (severe)(procedure related) None 1. Nausea- 20 2. Upper abdominal pain13 3. Pseudopolyp- 13 4. Implant site inflammation- 10 5. Vomiting- 6 1. Early device explantation(4/26)a.) Device migration/rotationi.) Symptomatic- 1/4 (3m post implant) ii.) Asymtpomatic- 1/4 (discovered on scheduled endoscopy, 4 m post implant) b.) Epigastric pain- 1/4 (3m post implant) c.) Nausea and vomiting- 1/4 (sleeve obstruction, 1 wk post implant) N/A N/A N/A None DNA None Peri-procedure complication 24 weeks 1 year 5 S.No Author/ Year/ Location Device/Int ervention Number of subjects Technique Duration of intervention Peri-procedure complication Minor adverse events (self limited/ mild -moderate) (procedure related) Major adverse events (severe)(procedure related) DJBS implant 28 DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system 1 year None DNA None 24 weeks DNA DNA DNA 6 months DNA DNA DNA 11 De Jonge et al. 2013, Netherlands [15] DJBS implant 17 DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system 12 De Moura et al. 2011, Brazil [16] DJBS implant Enrolled- 81 Successfully implanted78 DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system 6 S.No 13 Author/ Year/ Location De Moura et al, 2011, Brazil [17] Device/Int ervention DJBS implant Number of subjects 22 Technique DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system Duration of intervention 52 week 7 Peri-procedure complication 1. Nausea- 10/22 (45.5%) 2. Vomiting- 7/22 (31.8%) Minor adverse events (self limited/ mild -moderate) (procedure related) Major adverse events (severe)(procedure related) 1. GI complaints- 21/22 (95.5%) a.) Abdominal distention3/22 (13.6%) b.) Abdominal pain upper20/22 (90.9%) c.) Diarrhea- 3/22 (13.6%) d.) Nausea- 11/22 (50%) e.) Vomiting- 14/22 (63.6%) 1. Early device explantation (9/22)a.) Device migration/rotation- 3/9 at 36,36 and 48 wks post implant b.) GI bleeding- 1/9 at 4 weeks post implant c.) Abdominal pain- 2/9 at 21 and 30 wk post implant d.) Subject non compliance with follow up- 2/9 at 20 and 32 wks post implant e.) Metastatic ovarian cancer- 1/9 unrelated to device, 17 weeks post implant. S.No 14 Author/ Year/ Location Tarnoff et al. 2008 U.S.A, Chile, Brazil [18] Device/Int ervention Number of subjects Technique Duration of intervention Peri-procedure complication Minor adverse events (self limited/ mild -moderate) (procedure related) DJBS implant and low fat diet Enrolled- 26 Successfully implanted25 DJBS implant was delivered with help of endoscope and fluoroscopy using wire catheter system 12 wks Noncardiac chest pain- 1/25 1. GI tract Hemorrhage1/25 2. Nausea- 6/25 3. Abdominal pain- 15/25 4. Vomiting- 8/25 5. Abdominal distention11/25 6. Constipation- 1/25 7. Epigastric discomfort1/25 Low fat diet alone 14 N/A 12 wks N/A N/A Abbreviations: DJBS- Duodenojejunal bypass sleeve DNA- Data not available EGD- Esophagogastroduodenoscopy GDJBS- Gastroduodenojejunal bypass sleeve GI- Gastrointestinal N/A- Not applicable 8 Major adverse events (severe)(procedure related) 1. GI tract Hemorrhage- 3/25 2. Nausea- 1/25 3. Abdominal pain- 1/25 5/25 subjects underwent early device explantation- 1. GI bleed at 12-17 day-3/5 2. Sleeve obstruction at 30th day1/5 3. Device migration at 47th day1/5 N/A